September 8, 2023

CHICAGO — Visioneering Technologies Inc. completed a six-year clinical trial for its NaturalVue Multifocal (MF) contact lenses, and now, the company has packaged three independent studies to show the consistency of the results.
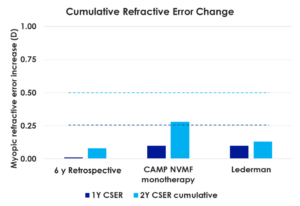
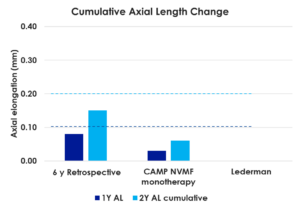
The analysis, entitled “Consistency in Outcomes — Results from Three Different Retrospective Analyses,” featured findings across 108 children wearing NaturalVue Multifocals and was presented by Douglas P. Benoit, OD, FAAO, Executive Director, Medical Affairs for VTI, at the Vision By Design meeting held in Chicago, Illinois. Average annual refractive error change in each study was 0.15D or less at years one and two, which is significantly less than predicted in age- and ethnicity-matched virtual control subjects. Axial length data was available for two cohorts and was found to have increased, on average, less than 0.10 mm annually at years one and two (approximately the amount anticipated in emmetropic children). The CARE value (cumulative absolute reduction in axial elongation) versus age- and ethnicity-matched virtual control subjects in these cohorts were significant and comparable (0.17 mm at year one and 0.32 mm at year two).
 |
 |
The three independently conducted studies included:
- NaturalVue Monotherapy Subgroup Analysis from The CAMP study (The Clinical Algorithm for Myopia Progression) conducted by Treehouse Eyes
- Myopia Control with Extended Depth of Focus Multifocal Contact Lenses, Carolyn R. Lederman, MD & Edward S. Harkness Eye Institute, Vagelos College of Physicians and Surgeons, Columbia University Irving Medical Center, New York, NY (Presented as a POSTER at the 2023 American Association for Pediatric Ophthalmology and Strabismus 48th Annual Meeting, 29 March-02 April 2023)
- Subgroup Analysis from NaturalVue 6-year Retrospective data: Cooper J, O’Connor B, Aller T, et al. Reduction of myopic progression using a multifocal soft contact lens: A retrospective cohort study. Clin Ophthalmol. 2022 Jul; 16:2145-2155.
Axial length change was measured for a subset of the study subjects.* Significantly, the average axial elongation change was approximately 0.10 mm per year through 47 months of follow-up, which approximates that expected for a non-myopic child of a similar age range1 and shows that NaturalVue MF contact lenses help to reduce the anatomical cause of myopia progression.2 To be able to compare the effectiveness of NaturalVue MF to changes observed in children not wearing the lenses, an age- and ethnicity-matched virtual control group (N=188) developed from 63 randomized clinical trials was used.2,3 This analysis demonstrated a Cumulative Absolute Reduction of axial Elongation (termed a “CARE” value) of 0.44 mm less axial elongation over three years for NaturalVue MF than would be expected for age- and ethnicity-matched children.1 The use of the CARE value enables ECPs the ability to compare data from one study to another more easily. The CARE value for NaturalVue MF compares very favorably to those of other myopic progression interventions such as orthokeratology lenses, with a CARE value of 0.44 mm over seven years, or other soft contact lenses with a CARE value of 0.30 mm at three years.1
To provide the eye care community and potential corporate partners with additional data, VTI will soon report preview data from the one-year interim results of the PROTECT study, the international multi-centered (U.S., Canada, Hong Kong, and Singapore) double-blinded, randomized, and controlled study of NaturalVue MF in myopic children.
“Despite having vastly different demographics, with sites spread across America, the results in these three studies were remarkably similar,” said Dr. Ashley Tuan, OD, PhD, VTI’s Chief Medical Officer. “This demonstrates that real-world results with this unique design are consistent and repeatable.”
References
*CAMP Study Funding/Support: Funding for this project was provided in part by Euclid Systems Corporation and Visioneering Technologies, Inc., through unrestricted grants. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or the decision to submit the manuscript for publication.
- Cooper J, O’Connor B, Aller T, Dillehay SM, Weibel K, Benoit D. Reduction of Myopic Progression Using a Multifocal Soft Contact Lens: A Retrospective Cohort Study. Clin Ophthalmol. 2022 Jul 4;16:2145-2155. doi: 10.2147/OPTH.S370041. PMID: 35814919; PMCID: PMC9270009.
- Brennan NA, Toubouti YM, Cheng X, Bullimore MA. Efficacy in myopia control. Prog Retin Eye Res. 2021;83:100923. doi:10.1016/j. preteyeres.2020.100923
- Brennan NA. Why “CARE” for myopia? Review of myopia management; October 1, 2020.













