December 1, 2021
By Professor Padmaja Sankaridurg, PhD, Head, Myopia Program and Head, Intellectual Property at the Brien Holden Vision Institute
Although the design features and the efficacy vary between the various lens types, the overall evidence for myopia control with soft CLs is robust, clinically significant, and supported by well-conducted clinical trials.

There is an ever-increasing availability of soft contact lenses that slow myopia progression. The platform has evolved from “off-label” use of multifocal or multifocal-like lenses to contact lenses (CLs) specifically marketed for myopia control, such as the MiSight 1 day (CooperVision, San Ramon, Calif.), MYLO (Mark’Ennovy, Spain), NaturalVue Multifocal 1 Day (Visioneering Technologies, Alpharetta, Ga.), DISC-1Day (Hong Kong Polytechnic University and Vision Science and Technology Co, Hong Kong), Relax (SwissLens, France), and Esencia (Eurolent servicios Opticos S.L) lenses. Many of these CLs have regulatory approvals in one or more countries with indications for myopia control. Recent media announcements point to more lenses on the horizon.1 Although the design features and the efficacy vary between the various lens types, the overall evidence for myopia control with soft CLs is robust, clinically significant, and supported by well-conducted clinical trials.
In translating evidence to practice, the requirement of “significant myopia control” remains a key determinant. However, other important factors influence and guide successful implementation and management of myopia control. These are related to visual performance, safety and ocular health with lenses, rebound on discontinuation, and patient-related factors, such as motivation, compliance to lens wear, practitioner education, and experience with myopia control strategies, cost, and product availability. With the field rapidly evolving, some of these factors are being addressed in clinical studies/trials, and keeping abreast of the evidence may inform management approaches to myopia control.
Visual Performance and Patient Satisfaction
In a clinical trial where two types of myopia control CLs were assessed for initial wear experience, more than half of the participants preferred one of the two lens types, with the preferred lens varying between individuals. Therefore, it was recommended that, where possible, practitioners should fit patients with more than one myopia control CL type to determine a preference as improved satisfaction may reduce the risk of dropouts.2 In the trial, central vision clarity and comfort were associated with overall satisfaction. Although objective visual acuity (VA) measurements, such as distance and high and low contrast VA with multifocal CLs are comparable to VA with single vision lenses, subjective assessments indicate lower scores for overall visual quality as well as subjective symptoms of halos and ghosting. The presence and/or severity of these symptoms vary depending on lens type, lens fit, ADD power, and pupil diameter.2-8 Additionally, to achieve the best VA with Biofinity Multifocal “D” lenses, most children required an over-refraction of between -0.50D and -0.75D. Although more minus was required to optimize central VA, it was said that the lens continued to impose myopic defocus at most points across the peripheral retina.6
Children who are non-adherent to lens wear are more likely to discontinue, provide lower ratings for visual quality and ocular comfort (see Figure 1), are more likely to be males, and have lower baseline myopia, lower higher contrast VA, and esophoria.9 A better myopia control efficacy was observed with greater compliance/adherence to lens wear in some trials but was not found to be related to compliance in another trial.10-12
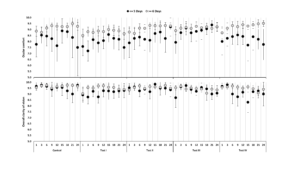
Figure 1: Overall comfort and clarity of vision in children categorized as non-adherent/adherent to lens wear based on days of wear/week. With permission from Weng et al. CLAE, 44(10), p94-101, 2021
The above data indicate that optimizing visual performance in myopia control CLs, paying attention to subjective feedback, and ensuring satisfaction in their chosen modality at the start is likely to promote wearer compliance, reduce dropouts, and likely improve efficacy.
Efficacy
In the BLINK trial, both medium (+1.50D) and high add (+2.50D) center distance multifocals effectively slowed myopia compared to a single vision CL. However, efficacy was greater with the higher ADD lens.10 Therefore, when contemplating a center-distance multifocal, a higher add power (+2.50D) may be relevant for younger, at-risk individuals, but be aware that higher add powers in multifocal CLs may impact visual performance. Interestingly, in a two-year randomized clinical trial involving four test CLs (two lenses based on extended depth of focus technology and two multifocal lenses with plus power across both central and peripheral zones), all CLs slowed myopia with no difference in progression between the various lens designs.13 Furthermore, in a short-term cross-over clinical dispensing trial involving MiSight and EDOF lenses, both lenses demonstrated similar efficacy (O-049, pg. 63, IMC 2019 proceedings, accessed Nov. 25, 2021).
A recent addition to the growing list of myopia control CLs is a custom-made, lathe cut soft CL with a peripheral progressive +2.00D ADD on the front surface. It is reported that the CL presents a central flattening and peripheral steepening on the back surface (referred to as reverse geometry soft CL). In a randomized trial, the lens slowed myopia in children aged 7-15 yrs. The details are scant, but the reverse geometry features were considered to aid in lens stability, provide continuous peripheral defocus with no impact on corneal power.14
Ocular Health, Safety, and Rebound on Discontinuation
In children, long-term (six years) wear of myopia control CLs had minimal impact on ocular physiology.15 A retrospective review found that in children (8 to 16 years of age) wearing soft CLs, the incidence of significant adverse events such as microbial keratitis and other inflammatory events are comparable to rates observed in adults.16 With the Esencia trial, although lenses were used on a daily disposable basis, there were some incidents of corneal neovascularisation — such events are unusual with modern soft lenses and require further exploration.14 In the seventh year of the multi-center MiSight trial, participants were switched from MiSight lenses to single vision lenses. Myopia was reported to have progressed per anticipated age normal levels with no acceleration in progression or “rebound.” Similarly, in another study with a smaller sample size, myopia progression in children discontinued from MiSight lens wear was similar to that observed with those wearing single vision spectacles.17
| Key points from recent evidence: |
|
|
|
|
 |
Professor Padmaja Sankaridurg, PhD, is Head, Myopia Program and Head, Intellectual Property at the Brien Holden Vision Institute and a Conjoint Professor at the School of Optometry and Vision Science, Sydney, Australia. She serves on the Advisory board of the International Myopia Institute and Review of Myopia Management. |
References
- Johnson & Johnson Vision’s ACUVUE Abiliti 1-Day Soft Therapeutic Lenses for Myopia Management Receive Approval in Canada. 2021: Reviewofmm.com. p. https://reviewofmm.com/johnson-johnson-visions-acuvue-abiliti-for-myopia-management-receives-approval-in-canada/.
- Ghorbani-Mojarrad, N., et al., Patient experience and physiological response to two commercially available daily disposable myopia control contact lenses. Contact Lens and Anterior Eye, 2021.
- Huang, X., et al., Visual quality of juvenile myopes wearing multifocal soft contact lenses. Eye and Vision, 2020. 7(1): p. 41.
- Kollbaum, P.S., et al., Vision performance with a contact lens designed to slow myopia progression. Optom Vis Sci, 2013. 90(3): p. 205-14.
- García-Marqués, J.V., et al., Comparison of short-term light disturbance, optical and visual performance outcomes between a myopia control contact lens and a single-vision contact lens. Ophthalmic Physiol Opt, 2020. 40(6): p. 718-727.
- Schulle, K.L., et al., Visual Acuity and Over-refraction in Myopic Children Fitted with Soft Multifocal Contact Lenses. Optom Vis Sci, 2018. 95(4): p. 292-298.
- Bickle, K.M., G.L. Mitchell, and J.J. Walline, Visual Performance with Spherical and Multifocal Contact Lenses in a Pediatric Population. Optom Vis Sci, 2021. 98(5): p. 483-489.
- Rizzo, G.C., et al., Centration assessment of an extended depth of focus contact lens for myopic progression control. Cont Lens Anterior Eye, 2021: p. 101533.
- Weng, R., et al., Exploring non-adherence to contact lens wear schedule: Subjective assessments and patient related factors in children wearing single vision and myopia control contact lenses. Cont Lens Anterior Eye, 2021. 44(1): p. 94-101.
- Walline, J.J., et al., Effect of High Add Power, Medium Add Power, or Single-Vision Contact Lenses on Myopia Progression in Children: The BLINK Randomized Clinical Trial. Jama, 2020. 324(6): p. 571-580.
- Sankaridurg, P., et al., Myopia control with novel central and peripheral plus contact lenses and extended depth of focus contact lenses: 2 year results from a randomised clinical trial. Ophthalmic Physiol Opt, 2019. 39(4): p. 294-307.
- Lam, C.S.Y., et al., Defocus Incorporated Multiple Segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomised clinical trial. Br J Ophthalmol, 2019.
- Sankaridurg, P., et al., Myopia control with novel central and peripheral plus contact lenses and extended depth of focus contact lenses: 2 year results from a randomised clinical trial. Ophthalmic Physiol Opt, 2019. 39(4): p. 294-307.
- Garcia-Del Valle, A.M., et al., Efficacy and safety of a soft contact lens to control myopia progression. Clin Exp Optom, 2021. 104(1): p. 14-21.
- Woods, J., et al., Ocular health of children wearing daily disposable contact lenses over a 6-year period. Cont Lens Anterior Eye, 2021. 44(4): p. 101391.
- Chalmers, R.L., et al., Adverse event rates in the retrospective cohort study of safety of paediatric soft contact lens wear: the ReCSS study. Ophthalmic Physiol Opt, 2021. 41(1): p. 84-92.
- Ruiz-Pomeda, A., et al., Rebound Effect in the Misight Assessment Study Spain (Mass). Curr Eye Res, 2021: p. 1-4.













