April 15, 2024
By Dwight Akerman, OD, MBA, FAAO, FBCLA, FIACLE
Topical low-concentration atropine eye drops to help slow the progression of juvenile-onset myopia is an area of active research, as questions remain regarding optimal dosing, efficacy, rebound, and length of treatment. The Low Concentration Atropine for Myopia Progression (LAMP) Study Phase 3 Report reported that during the third year continued atropine treatment achieved a better effect across all concentrations compared with the washout regimen. A concentration of 0.05% atropine remained the optimal concentration over three years in Chinese children. The differences in rebound effects were clinically small across all three studied atropine concentrations. Stopping treatment at an older age and lower concentration are associated with a smaller rebound.
The purpose of the Five-Year Clinical Trial of Low-concentration Atropine for Myopia Progression (LAMP) Study: Phase 4 Report was to evaluate (1) the long-term efficacy of low-concentration atropine over five years, (2) the proportion of children requiring retreatment and associated factors, and (3) the efficacy of pro re nata (PRN) retreatment using 0.05% atropine from year three to five.
Children aged 4–12 years originally from the LAMP study were followed up for five years. During the third year, children in each group originally on 0.05%, 0.025%, and 0.01% atropine were randomized to continued treatment and treatment cessation. During years four and five, all continued treatment subgroups were switched to 0.05% atropine for continued treatment, while all treatment cessation subgroups followed a PRN retreatment protocol to resume 0.05% atropine for children with myopic progressions of 0.50D or more over one year. Generalized estimating equations were used to compare the changes in spherical equivalent (SE) progression and axial length (AL) elongation among groups.
Over five years, the cumulative mean spherical equivalent (SE) progressions were -1.34±1.40D for the initial 0.05% atropine group, -1.97±1.03D for the initial 0.025% atropine group, and -2.34±1.71D for the initial 0.01% atropine group. Axial length elongation over the five years displayed a similar trend among groups. Among the PRN retreatment group, 87.9% (94/107) of children needed retreatment. The proportion of retreatment across all studied concentrations is similar.
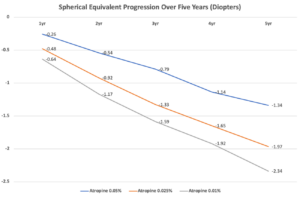
The original LAMP Study found that 0.05% atropine was more effective at slowing myopia progression than 0.025% or 0.01% formulations. Now, a follow-up study on some of the same participants shows that 0.05% atropine is effective at five years, with similar outcomes among children who had initially used other concentrations. Click image to enlarge.
The researchers concluded that over five years, the continued 0.05% atropine treatment demonstrated good efficacy for myopia control. Most children needed to restart treatment after atropine cessation at year three. Restarted treatment with 0.05% atropine achieved similar efficacy as continued treatment. Children should be considered for retreatment if myopia progresses after treatment cessation.
Abstract
Five-Year Clinical Trial of Low-concentration Atropine for Myopia Progression (LAMP) Study: Phase 4 Report
Xiu Juan Zhang, Yuzhou Zhang, Benjamin H K Yip, Ka Wai Kam, Fangyao Tang, Xiangtian Ling, Mandy P H Ng , Alvin L Young, Pei-Chang Wu, Clement C Tham, Li Jia Chen, Chi Pui Pang, Jason C Yam
Purpose: To evaluate (1) the long-term efficacy of low-concentration atropine over 5 years, (2) the proportion of children requiring retreatment and associated factors (3) the efficacy of pro re nata (PRN) retreatment using 0.05% atropine from year 3 to 5.
Design: A randomized, double-masked extended trial.
Methods: Children aged 4–12 years originally from the Low-Concentration Atropine for Myopia Progression study were followed up for 5 years. During the third year, children in each group originally on 0.05%, 0.025%, and 0.01% atropine were randomized to continued treatment and treatment cessation. During years 4 and 5, all continued treatment subgroups were switched to 0.05% atropine for continued treatment, while all treatment cessation subgroups followed a PRN retreatment protocol to resume 0.05% atropine for children with myopic progressions of 0.5D or more over one year. Generalized estimating equations were used to compare the changes in spherical equivalent (SE) progression and axial length (AL) elongation among groups.
Outcomes Measures: (1) Changes in SE and AL over 5 years in different groups over 5 years; (2) Proportion of children who needed retreatment; (3) Changes in SE and AL in continued treatment and PRN retreatment groups from years 3 to 5.
Results: 269 (82.5%) of 326 children from the third year completed 5 years of follow-up. Over 5 years, the cumulative mean SE progressions were –1.34±1.40D, –1.97±1.03D, and –2.34±1.71D for the continued treatment groups with initial 0.05%, 0.025%, and 0.01% atropine respectively (P=0.02). Similar trends were observed in AL elongation (P=0.01). Among the PRN retreatment group, 87.9% (94/107) of children needed retreatment. The proportion of retreatment across all studied concentrations is similar (P=0.76). The SE progressions for continued treatment and PRN retreatment groups from years 3 to 5 were – 0.97D±0.82D, and –1.00±0.74D (P=0.55), and the AL elongations were 0.51±0.34mm, and 0.49±0.32mm (P=0.84), respectively.
Conclusions: Over 5 years, the continued 0.05% atropine treatment demonstrated good efficacy for myopia control. The majority of children needed to restart treatment after atropine cessation at year 3. Restarted treatment with 0.05% atropine achieved similar efficacy as continued treatment. Children should be considered for retreatment if myopia progresses after treatment cessation.
Zhang, X. J., Zhang, Y., Yip, B. H., Kam, K. W., Tang, F., Ling, X., … & Yam, J. C. (2024). Five-Year Clinical Trial of Low-concentration Atropine for Myopia Progression (LAMP) Study: Phase 4 Report. Ophthalmology. 2024 Mar 15. Epub ahead of print.
DOI: https://doi.org/10.1016/j.ophtha.2024.03.013