All FDA Articles
-
 22Informed Consent
22Informed ConsentWhy Different FDA Indications Matter
April 15, 2024 Andrew Neukirch, OD The relationships my team and I cultivate with parents is built on trust. It’s my responsibility...
-

 556Latest Myopia News
556Latest Myopia NewsSightGlass’ DOT Spectacle Lenses Receive FDA Breakthrough Device Designation
February 14, 2024 LOS ALTOS, Calif. — SightGlass Vision’s Diffusion Optics Technology (DOT) spectacle lenses have received Breakthrough Device designation from the...
-
 1.2KClinical
1.2KClinicalThe Importance of Measuring Axial Length in Myopia Management
January 1, 2024 By Thomas Naduvilath, Msc Biostatistics, PhD, Head of Biostatistics at BHVI With the emphasis of myopia control to reduce axial...
-
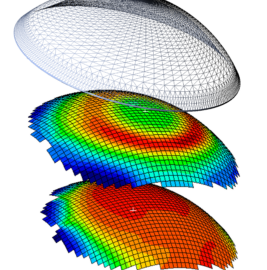
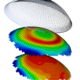 488Clinical
488ClinicalAccuLens’ NewVision Corneo-Scleral Lens Provides Customized Fit, Patient Comfort
sponsored content November 15, 2023 By Nicholas Gidosh, OD AccuLens’ NewVision corneo-scleral orthokeratology lens has received FDA clearance for daily wear. The...
-
 1.0KLatest Myopia News
1.0KLatest Myopia NewsCooperVision Launches Consumer Marketing Campaign Ahead of Back-to-School Season
July 13, 2023 SAN RAMON, Calif. — At this year’s Vision Expo East event, CooperVision announced its latest myopia management campaign, “Make...
-
 932Latest Myopia News
932Latest Myopia NewsFDA Accepts Vyluma’s New Drug Application for NVK002 Atropine
June 6, 2023 BRIDGEWATER, N.J. — The U.S. Food and Drug Administration (FDA) has accepted Vyluma’s new drug application (NDA) for its...
-

 2.5KClinical
2.5KClinicalLeading OD Prescribes MiSight To Slow Own Daughter’s Myopia Progression
sponsored content April 18, 2023 Hannah Yecheskel Neubauer, OD, has been in the field for more than 20 years and was named...
-
 1.6KLatest Myopia News
1.6KLatest Myopia NewsiVeena Completes Pre-IND FDA Meeting
November 11, 2021 SALT LAKE CITY — iVeena Delivery Systems announced that the company has successfully completed a pre-IND (Investigational New Drug)...
-

 3.5KLatest Myopia News
3.5KLatest Myopia NewsEssilor’s Stellest Lenses Receive FDA Breakthrough Device Designation
May 17, 2021 CHARENTON-LE-PONT, France — The U.S. Food and Drug Administration (FDA) granted Essilor’s Stellest lenses the Breakthrough Device designation. With...
-

 5.8KLatest Myopia News
5.8KLatest Myopia NewsJohnson & Johnson Vision’s ACUVUE Abiliti OrthoK Receives FDA Approval
May 12, 2021 JACKSONVILLE, Fla. — The U.S. Food and Drug Administration (FDA) has approved ACUVUE Abiliti Overnight Therapeutic Lenses, Johnson &...
-
 8.2KLatest Myopia News
8.2KLatest Myopia NewsJohnson & Johnson Vision Receives FDA’s Breakthrough Device Designation for Myopia Control Lens
July 17, 2020 Johnson & Johnson Vision has received FDA’s Breakthrough Device designation for its myopia control lens, Chris DelOrefice, Johnson &...
-

 4.2KPatient Communication
4.2KPatient CommunicationExplaining “Off Label” to Parents
Balance the risks of using an off-label treatment with the eye health consequences of no treatment at all.
-

 2.5KLatest Myopia News
2.5KLatest Myopia NewsMyopia Management Focus of Pipeline Expansion for Leo Lens Pharma
SAN DIEGO – Leo Lens Pharma has received a notice of allowance from the U.S. Patent and Trade Office for a patent...
-

 5.9KEditor’s Perspective
5.9KEditor’s PerspectiveMiSight® 1 Day FDA Approval: Why It Matters
MiSight® 1 day’s FDA approval is an important step forward in stopping the myopia epidemic facing American children.
-

 3.6KLatest Myopia News
3.6KLatest Myopia NewsC&E GP Specialists Inc. Awarded ISO 13485:2016 Certificate
Nov. 15, 2019 San Diego, Calif. – C&E GP Specialists Inc., a leading manufacturer of orthokeratology specialty rigid gas-permeable contact lenses, has...
