August 5, 2021
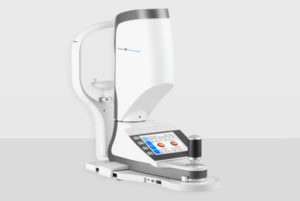 ARLINGTON, Wash. — OCULUS announced that the Myopia Master has received FDA approval.
ARLINGTON, Wash. — OCULUS announced that the Myopia Master has received FDA approval.
The Myopia Master combines the most important parameters for myopia management: refraction, axial length measurement, and keratometry. The quick, contactless, and accurate measurement method for axial length is not influenced by the accommodation-status of the eye and is performed using interferometry.
“We are very happy to announce that we just received FDA clearance for the Myopia Master!” said Christian Kirchhübel, CEO of OCULUS Optikgeräte GmbH. “Two years ago, we presented the first device to the public at the Opti in Munich. Since then, we have been working with BHVI (outside the U.S.) to not only deliver the measurements required but also the metrics scales. The new Myopia Master can replace your standard ARK so that not another spot is needed in the crowded examination room. “













