sponsored content
August 16, 2021
By Shalu Pal, OD, FAAO, FSLS, FBCLA
Our obligation as health care providers is to prescribe myopia management during a child’s growing years.
 In December of 2001, the United States Congress passed the No Child Left Behind Act requiring public elementary and secondary schools to measure their students’ performance relative to developmental norms and age expectations. But, as the name implies, there was a special focus on disadvantaged children in an attempt to bring them up to a proficient level once identified. Many issues were present with this Act, and it was replaced in December 2020 by the Every Student Succeeds Act. With this shift, the new underlying theme is to ensure that each and every child be given a chance to succeed, to find those struggling, and to help them through a system where they may once have gotten lost.
In December of 2001, the United States Congress passed the No Child Left Behind Act requiring public elementary and secondary schools to measure their students’ performance relative to developmental norms and age expectations. But, as the name implies, there was a special focus on disadvantaged children in an attempt to bring them up to a proficient level once identified. Many issues were present with this Act, and it was replaced in December 2020 by the Every Student Succeeds Act. With this shift, the new underlying theme is to ensure that each and every child be given a chance to succeed, to find those struggling, and to help them through a system where they may once have gotten lost.
Children are vulnerable young people who potentially may be negatively impacted when evaluated and tested in clinical research. Institutional Review Boards must take extra care to protect children as participants, particularly when the scope is beyond observational, and when an intervention is involved. Consent for the child and assent from the parent are among the many additional requirements. Participation of children as research subjects must also be justified. One of the changes within the new Every Student Succeeds Act is that parents are given the option to opt out of testing.
Understanding the Efficacy of Treatments

Dr. Shalu Pal & Associates, Toronto.
In the context of myopia management, enrolling children with their parental consent in such studies is assuredly justified considering the eye health implications with every additional diopter of myopia.1 CooperVision undertook and recently completed a seven-year study on children who were 8 to 12 years of age at the initiation of treatment. Children wearing MiSight 1 day progressed less than 1.00 D on average over the six years of treatment.2 This remains the longest prospective, longitudinal study of children in contact lenses and gives us the ability to understand MiSight 1 day’s myopia control effects, as so much of the learnings from the results are directly applicable to clinical care. We learned that slowing of myopia with a safe optical device is possible in age-appropriate children and practical to use in clinical practice.
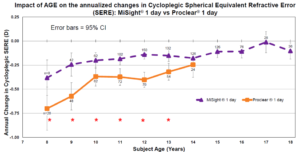
A common question I am asked by parents and practitioners alike is, “How do I know that my treatment is working?” Well, the data in the MiSight study outlines what is expected for annual progression rates by age, with and without treatment.3 By following myopic patients — those with myopic correction alone and those with myopic management treatments — every six months, we can compare their progression to age norms and determine if they fall within a range of what is expected. This then allows us to better educate our patients’ parents and adjust treatment plans and follow-up schedules appropriately. That being said, all patients present with their own set of risk factors, lifestyle habits, parental concerns, and level of motivation. Each child is unique and with that their treatment strategy must also be as well.
Long-Term Benefits of Myopia Management
I have seen it time and again where an unsettled child comes into the office and finds calm energy once given the correct prescription. But we live in a day and age when treating a prescription for vision alone is just not enough. Children with myopia deserve to have a chance to improve their visual future fate. Reducing their myopia by 1.00 D has the ability to reduce the future risk of myopic maculopathy by 40%.1 A lower myopic prescription results in better uncorrected vision, which can improve daily activities such as sports, as well as functioning in the home without correction before bed and in the mornings.
Pediatric optometrists will say that children are the most underserved patient population in eye care. They are often not brought in for exams by their parents; frequently, if they are, they may not be able to explain their lack of vision due to a sheer unknowing of what great vision can be. Every day, we have a decision to make with every patient encounter. What could be more rewarding than to be a part of each child’s story in how they were able to soar to new heights with the ocular benefits of contact lenses and potentially lower levels of myopia throughout life? I’d say that’s a wonderful privilege to have, and our obligation as health care providers is to prescribe myopia management during a child’s growing years. The time is now and no child deserves to be left behind.

Shalu Pal, OD, FAAO, FSLS, FBCLA, started a group practice in Toronto, specialising in advanced specialty contact lens fitting, myopia management, dry eye management, concussion rehabilitation, vision therapy, and ocular aesthetics. She strives for the betterment of the profession through education and motivation. This article is sponsored by CooperVision.
Indications for use: MiSight® 1 day (omafilcon A) soft (hydrophilic) contact lenses for daily wear are indicated for the correction of myopic ametropia and for slowing the progression of myopia in children with non-diseased eyes, who at the initiation of treatment are 8-12 years of age and have a refraction of -0.75 to -4.00 diopters (spherical equivalent) with ≤ 0.75 diopters of astigmatism. The lens is to be discarded after each removal.
References:
- Bullimore MA, Brennan NA. Myopia Control: Why Each Diopter Matters. Optom Vis Sci. 2019 Jun;96(6):463-465. doi: 10.1097/OPX.0000000000001367. PMID: 31116165.
- Chamberlain P, Arumugam B, Jones D et al. Myopia Progression in Children wearing Dual-Focus Contact Lenses: 6-year findings. Optom Vis Sci 2020;97(E-abstract): 200038
- Arumugam B, Chamberlain P, Bradley A et al. The Effects of Age on Myopia Progression with Dual-Focus and Single Vision Daily Disposable Contact Lenses. Optom Vis Sci 2020;97(E-abstract):205340, AAO 2020 Poster.