sponsored content
January 17, 2022
By Justin Kwan, OD, FAAO
As we enter 2022, more myopia science from this landmark study will continue to be discovered and shared.

The MiSight 1 day study remains the longest continuous soft contact lens study for myopia control following age-appropriate children for seven years. There were periods when kids were assigned either the treatment of dual focus MiSight 1 day or the single vision correction of Proclear 1 day. The journey of these research participants during their growing years is very reflective of patients presenting for optometric care in the following ways:
- Half of the children were started on myopia control with MiSight 1 day between the ages of 8 to 12 years old.
- Half of the children were started on myopia control later, between the ages of 11 to 15 years old, after their myopia may have progressed to higher levels (original control group).
- All of the children had their myopia control stopped in their mid to late teenage years, starting between the ages of 14 to 18, and concluding year seven of the study between ages 15 to 19.
- Children may receive between three and six years of myopia control with MiSight 1 day, where every year of use halves the progression of myopia, on average1 — age is the strongest determinant of annual progression rates.2
Breaking Down the Findings
An ARVO poster presented by Chamberlain et al. in 20213 validates the MiSight study data points against a White and Asian model created by Brennen et al. using data from 63 studies.4 At the start of year four, the ethical thing to do was to provide the original control group with the treatment of MiSight. In doing so, the control group was lost, but the model estimates the treatment effect. Whether the children were treated with MiSight for three years or six years, the treatment effect was about 0.21mm savings in axial length elongation during years four to six.
Another ARVO poster presented by Arumugam et al. in 20211 looked more carefully at the average response to MiSight when broken down by age. It’s a good reminder that the younger they are, the faster they progress.2 And considering the age of enrollment and length of follow-up in the study, participants were as young as 8 and as old as 18 upon completion of year six. To point number four above, every year of MiSight treatment, when stratified by age, amounts to a 46% to 49% treatment effect. The cumulative treatment effect across all ages in this 10-year dataset of 8 to 18 was 0.87 mm. This corresponds to >2.00D of myopia control effect or savings when the previous ceiling was thought to be about 1.00D.5
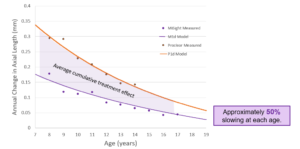
Arumugam, B et al. Modelling Age Effects of Myopia Progression for the MiSight 1 day Clinical Trial. Invest. Ophthalmol. Vis Sci. 2021;62(8):2333
Lastly, the original control group that received MiSight treatment later gives us the unique opportunity to study three years of pre-treatment myopia progression compared to three years of in-treatment myopia progression. Brennan and colleagues examined the Singapore Cohort Study of the Risk Factors for Myopia (SCORM) data. They found that these young children with myopia could have a certain progression rate in one year that correlated with their progression rate in the following year, but as a single factor, cannot fully predict subsequent year or long-term myopia progression.6 There was an inconsistent follow-up length, and the study was not one of intervention.
In contrast, the MiSight study was interventional and very structured. It demonstrated a proportional effect, meaning that the faster progressors benefited the most — they have the most slowing of their axial elongation relative to those years without MiSight. Many studies often report results using a cohort of ages and, while controlled, relegates the clinician to tell a parent expected results “on average.” Now, there is more specificity when looking at response rates in individual eyes broken down by age.
Looking to the Future
As we enter 2022, more myopia science from this landmark study will continue to be discovered and shared. Remember that parents value the efficacy of MiSight and the duration of the seven-year study.7 This is an eye health decision that also provides the immeasurable benefits of contact lens wear in children. MiSight recently celebrated two years of FDA approval on November 15, 2021, and remains the only contact lens with an indication for myopia control (QIT product code). Over 95% of children with myopia get worse during their growing years8 — they need your help now.
References
- Arumugam, B et al. Modelling Age Effects of Myopia Progression for the MiSight 1 day Clinical Trial. Invest. Ophthalmol. Vis Sci. 2021;62(8):2333.
- Donovan L et al.. Myopia progression rates in urban children wearing single-vision spectacles. Optom Vis Sci. 2012 Jan;89(1):27-32.
- Chamberlain, P et al. Measured and Predicted Axial Elongation in the MiSight 1 day Clinical Trial – 6 year results. Invest. Ophthalmol. Vis Sci. 2021;62(8):2342.
- Brennan, N et al. Influence of Age and Race on axial Elongation in Myopic Children. AAO paper 2018.
- Brennan NA, Toubouti YM, Cheng X, Bullimore MA. Efficacy in myopia control. Prog Retin Eye Res. 2021 Jul;83:100923.
- Brennan, N et al. Annual myopia progression and subsequent year progression in Singaporean children. Invest. Ophthalmol. Vis. Sci. 2020;61(7):76.
- Decision Analysts parent research focus groups May 25-27, 2021.
- Chamberlain P, Peixoto-de-Matos SC, Logan NS, Ngo C, Jones D, Young G. A 3-year Randomized Clinical Trial of MiSight Lenses for Myopia Control. Optom Vis Sci. 2019 Aug;96(8):556-567.†
*Indications for use: MiSight® 1 day (omafilcon A) soft (hydrophilic) contact lenses for daily wear are indicated for the correction of myopic ametropia and for slowing the progression of myopia in children with non-diseased eyes, who at the initiation of treatment are 8-12 years of age and have a refraction of -0.75 to -4.00 diopters (spherical equivalent) with ≤ 0.75 diopters of astigmatism. The lens is to be discarded after each removal.
†The 3-year peer-reviewed paper was the recipient of the American Academy of Optometry’s Garland W. Clay Award. It has been cited over 70 times in a period of just two short years.















