sponsored content
April 17, 2023
By Monica Jong, BOptom, PhD, Global Director of Professional Education, Myopia, Johnson & Johnson Vision
 There has been an explosion of myopia research in recent years, which is great news for clinicians and patients alike. While emerging research forms the basis of clinical treatments, the strength of different research methodologies varies greatly.1 Thus, understanding the quality of research is important when translating new research to clinical practice.2,3 It’s more important now than ever to understand myopia research to appropriately make clinical decisions for your patients.
There has been an explosion of myopia research in recent years, which is great news for clinicians and patients alike. While emerging research forms the basis of clinical treatments, the strength of different research methodologies varies greatly.1 Thus, understanding the quality of research is important when translating new research to clinical practice.2,3 It’s more important now than ever to understand myopia research to appropriately make clinical decisions for your patients.
Peer-Reviewed Publications
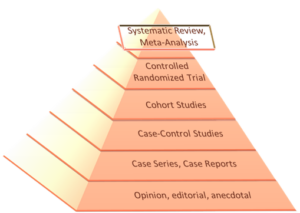
Scientific progress is largely recorded by publications in peer-reviewed journals, which provide the most trusted form of scientific communication. One way to assess scientific quality in such publications is by using the hierarchy of evidence (see Figure 14), where the highest quality methodologies are at the top and the lowest are at the bottom of the pyramid.
Peak of the Pyramid: Meta-Analysis and Systematic Reviews
Meta-analyses and systematic reviews are at the peak of the evidence pyramid because they combine multiple studies to interpret the legitimacy of results (Figure 1). However, design and execution of a meta-analysis still requires some personal judgment and expertise, potentially risking different types of researcher bias.5 For example, it requires the observer to select studies to be included based on guidelines.6 Generally, the best papers are published in the highest quality journals, which can be judged in part by their journal impact factor.
Second Highest Level of Evidence: Randomized Controlled Trials (RCT)
The backbone of a meta-analysis is well conducted randomized controlled trials, which are considered the gold standard for testing the efficacy of an intervention. Well-executed studies require large sample sizes, an unbiased control group, randomization, and double masking.7 Although, double masking can be challenging for some treatments such as orthokeratology. A robust statistical analysis plan is also expected.
Characteristics of a high quality RCT:7
- Large sample sizes
- An unbiased control group
- Randomization and double masking
- A robust statistical analysis plan
- Multi-site ethnically diverse population
- Recruitment independent of seasonal variation
As practitioners, we still need to assess whether a randomized controlled trial is robust, as even well-designed experiments may commonly produce “statistically significant” false positive results.8 Let’s look at just a few of the reasons below.
Study Design Limitations
Control Group
There are multiple challenges to following a control group for several years in myopia control studies. Investigators have tried using within-subject study designs to eliminate the need for a control group because of the challenges in not offering treatment over the duration of a long-term clinical study when there is demonstrated treatment effect. For example, contralateral eye and crossover studies of myopia control have been conducted. These approaches have not been fully established. “Before-and-after” studies have also been attempted where progression during treatment is compared to that before treatment; however, prior progression has poor predictive value for future progression.9,10 New approaches to myopia intervention trials are needed to overcome the control group problem. For example, is it ethical to withhold treatment to those who need it? There is also the increasing difficulty in retaining study participants in an untreated group when they are aware that they are in the untreated group and drop out of a study.11 It has been suggested that a virtual control group may be the future of clinical trials.11
Study Population
Study populations and their recruitment should also be considered carefully. Any given intervention seems to achieve the same absolute, but not percentage, treatment effect regardless of age, baseline parameters, progression rate and ethnicity in different populations.12 We should also take into consideration how we apply results of the sample population from a study to an individual patient for whom treatment is being considered.
Study Duration and Location
Data can be affected by both time and duration of recruitment as seasonal variation in progression is known13 and the COVID-19 pandemic was associated with higher myopia progression rates.14 The number of study sites that contribute to data in a clinical study is also an important factor, with single-center trials being more vulnerable to biases and less representative of diverse patient populations.15 Multi-site investigations will have a more heterogeneous sample of subjects and improve the generalizability (external validity) of results compared to single-site trials.
Data Dredging
Another concern to watch out for is “data dredging” (or “p-hacking”).16 This is persistent analysis of data in different ways until a statistically significant outcome is obtained, or mining of data in search of significant relationships in a dataset without necessarily having a specific hypothesis in mind.17 Post-hoc analysis is an accepted form of scientific inquiry, but observations made under such circumstances should be considered as exploratory data analysis, suitable for forming a hypothesis to be tested rather than confirmatory or proof of an effect.18 An example of this may be sub-group analysis. A well-designed experiment will determine an appropriate sample size to test a hypothesis with an adequate amount of statistical power and an appropriate probability of a chance occurrence (commonly 5%) for rejection of the null hypothesis.19 Sub-group analyses should be planned as part of the initial study design because further p-value testing, known as multiple comparisons, can dramatically increase the probability that a published finding is incorrect. Correction for repeat p-value testing is possible but not always used.
Pyramid Base: Case Reports, Opinions, and Anecdotes
The business of clinical conferences, trade magazines, and online videos, podcasts, and articles is alive and well in the field of myopia. Despite peer-reviewed publications being the de facto source of scientific knowledge,20 case reports, opinions, and anecdotal evidence may still have value. Successful myopia management requires a broad range of skills and a breadth of knowledge. Most of what practitioners want to hear would probably not be classified as “science” per se but often practical tips on how to incorporate myopia management into a practice and how to talk to children and their parents. But in this article, we are considering a specific part of the equation; that is, accessing the true scientific evidence base.
In conclusion, keeping up with the latest scientific advances in the myopia field may have its challenges, but understanding the fundamentals of high-quality experimental design and analysis is helpful in navigating the science. Staying informed by reading widely, attending practitioner and scientific meetings, talking to colleagues, and looking for replication of information across different presentations and articles is a good basis to help practitioners keep abreast of all the exciting developments in myopia.
 |
Dr. Monica Jong, PhD, BOptom, is Global Director of Professional Education, Myopia, Johnson and Johnson Vision; Honorary Fellow, School of Optometry and Vision Science, UNSW, Sydney, Australia. As Global Director of Professional Education, Myopia, at Johnson & Johnson Vision, she leads practitioner education initiatives around the world to support evidence-based myopia management. She was the former Executive Director of the International Myopia Institute, an organization she helped found and lead. In this role she led the development of white papers and key initiatives to bring consensus to the field of myopia management by bringing together leading researchers, clinicians, educators, policy-makers, and public health experts. Dr. Jong has published numerous peer-reviewed articles, co-created the first global online education program in myopia at the Brien Holden Vision Institute, and co-authored the WHO report on the Impact of Myopia and High Myopia. She was the former secretary of the Refractive Error Work Group of the International Agency for the Prevention of Blindness (IAPB) and contributed to position papers and advocacy initiatives in uncorrected refractive error. She has supervised a number of graduate students and speaks regularly at scientific and practitioner meetings in myopia and refractive error. |
References
- Lexis L, Julien B. Science and the scientific method. Ch 1 How to Do Science. 2022.
- Ioannidis JP. Why most published research findings are false. PLoS Med. Aug 2005;2(8):e124.
- Brennan NA, Cheng X. Commonly Held Beliefs About Myopia That Lack a Robust Evidence Base. Eye Contact Lens. Nov 7 2019;45:215-225.
- Murad MH, Asi N, Alsawas M, Alahdab F. New evidence pyramid. Evid Based Med. 2016 Aug;21(4):125-7.
- Greco T, Zangrillo A, Biondi-Zoccai G, Landoni G. Meta-analysis: pitfalls and hints. Heart Lung Vessel. 2013;5(4):219-25.
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [Internet source]. [accessed 2023 March 28]. http://prismastatement.org/PRISMAStatement/PRISMAStatement
- Brocklehurst P, Hoare Z. How to design a randomised controlled trial. Br Dent J. 2017 May 12;222(9):721-726.
- Colquhoun D. An investigation of the false discovery rate and the misinterpretation of p-values. R Soc Open Sci. Nov 2014;1(3):140216.
- Nixon AD, Cheng X, Toubouti YM, Bullimore MA, Brennan NA. We can’t predict future axial elongation in myopic children with confidence. Optom Vis Sci. 2020;97:eAbstract 205354.
- Mutti DO, Sinnott LT, Brennan NA, et al. The Limited Value of Prior Change in Predicting Future Progression of Juvenile-onset Myopia. Optom Vis Sci. May 1 2022;99(5):424-433.
- Bullimore MA, Brennan NA, Flitcroft DI. The future of clinical trials of myopia control. Ophthalmic Physiol Opt. 2023 Mar 10. doi: 10.1111/opo.13120. Epub ahead of print.
- Brennan NA, Toubouti YM, Cheng X, Bullimore MA. Efficacy in myopia control. Prog Retin Eye Res. Jul 2021;83:100923. doi:10.1016/j.preteyeres.2020.100923
- Gwiazda J, Deng L, Manny R, Norton TT; COMET Study Group. Seasonal variations in the progression of myopia in children enrolled in the correction of myopia evaluation trial. Invest Ophthalmol Vis Sci. 2014 Feb 4;55(2):752-8. doi: 10.1167/iovs.13-13029.
- Cyril Kurupp AR, Raju A, Luthra G, Shahbaz M, Almatooq H, Foucambert P, Esbrand FD, Zafar S, Panthangi V, Khan S. The Impact of the COVID-19 Pandemic on Myopia Progression in Children: A Systematic Review. Cureus. 2022 Aug 26;14(8):e28444. doi: 10.7759/cureus.28444.
- Chung KC, Song JW, Group. WS. A guide to organizing a multicenter clinical trial. Plast Reconstr Surg. 2010;126:515-523.
- Smith GD, Ebrahim S. Data dredging, bias, or confounding. BMJ. 2002 Dec 21;325(7378):1437-8.
- Andrade C. HARKing, Cherry-Picking, P-Hacking, Fishing Expeditions, and Data Dredging and Mining as Questionable Research Practices. J Clin Psychiatry. Feb 18 2021;82(1)
- Mark Rubin & Chris Donkin (2022): Exploratory hypothesis tests can be more compelling than confirmatory hypothesis tests. Philosophical Psychology, DOI:10.1080/09515089.2022.2113771
- Gaus W, Mayer B, Muche R. Interpretation of statistical significance—exploratory versus confirmative testing in clinical trials, epidemiological studies, meta-analyses and toxicological screening (using Ginkgo biloba as an example). Clin Exp Pharmacol. 2015;5:182-187.
- Kelly J, Sadeghieh T, Adeli K. Peer Review in Scientific Publications: Benefits, Critiques, & A Survival Guide. EJIFCC. Oct 2014;25(3):227-43.
PP2023OTH4580