November 1, 2020
By Thomas Aller, OD, FBCLA
Collaborator, BHVI
 With the increasing incidence of myopia all around the world, predictions of greatly increased prevalence by the year 20501 and the availability of multiple treatment approaches that have shown varying degrees of effectiveness in clinical studies,2 a time is fast approaching when the standard of care for childhood myopia is to prescribe safe and effective methods for controlling progression unless it is not possible for a particular patient.3
With the increasing incidence of myopia all around the world, predictions of greatly increased prevalence by the year 20501 and the availability of multiple treatment approaches that have shown varying degrees of effectiveness in clinical studies,2 a time is fast approaching when the standard of care for childhood myopia is to prescribe safe and effective methods for controlling progression unless it is not possible for a particular patient.3
It may be worthwhile to consider briefly the currently available treatments for myopia progression control to understand better how these devices or treatments might be improved in the future and to understand the need for new approaches.
It is widely acknowledged that increased outdoor time may delay the incidence of myopia,4,5 and this is valuable as myopia is known to increase rapidly in younger children. Before these findings can be leveraged into innovative treatments, one needs to decide if the beneficial effect of outdoor time emanates from light intensity, particular wavelengths, flat dioptric space, vitamin D, dopamine or pupil size.
Outdoor light intensity is dramatically higher than most indoor spaces,6 and the amount of energy required to duplicate those levels indoors and cool those spaces likely would make this approach unfeasible. Manipulating wavelengths of indoor lighting or digital devices or filtering eyeglasses or contact lenses to favor specific wavelengths could be easily achieved once it is decided which, if any, wavelengths might be beneficial. Unfortunately, the scientific literature often seems to conflict on this topic, depending on the species studied.7-9
Pupil size might also influence myopia progression either because larger pupils allow more peripheral defocus in orthokeratology,10 or perhaps in smaller pupils outdoors, leading to greater depth of focus and clearer retinal images. If a greater depth of focus might be beneficial, perhaps the new miotic presbyopic drops11 or biofeedback methods for voluntary pupil constriction at distance might have some effect.12
In another possible application for light, there is a clinical trial ongoing in China on the use of low-level red light therapy to improve the choroidal metabolic rate and circulation with the goal of reducing scleral hypoxia to reduce myopia progression. Promising early results from the investigator’s case series led to the trial, so stay tuned.
Results from Pharmaceutical Treatments
Pharmaceutical treatments for myopia have shown excellent results in the case of atropine eye drops in various dosages.13 In most markets, low-dose atropine eye drops have to be sourced from a compounding pharmacy. However, there are currently three FDA Phase III clinical trials ongoing in the U.S. Nevakar is investigating two dosages of a stable, unit dose atropine and has recently executed a licensing agreement. Sydnexis is investigating deuterated water-based aqueous atropine drops. Eyenovia is studying low-dose atropine with a microdosing dispenser, just licensed by Bausch & Lomb.
With some promising results using oral 7-methylxanthine (7-MX), a metabolite of caffeine, to control myopia in children,14 studies began using topical caffeine eye drops.15 Perhaps a product extension for your favorite coffee shop one day?
Spectacle Lenses in Late Stages of Development
Spectacle lenses have been proposed and studied for about 50 years with results varying from possibly quite effective in the case of executive-style bifocals, but not commonly utilized,16 to barely effective in the case of progressive addition lenses,17 but widely prescribed. The beauty of a spectacle-based treatment for myopia control is that it can be used at the earliest stages of myopia progression, even in rapidly reducing hyperopia with an insufficient hyperopic buffer to avoid future myopia or pre-myopia.18 Unless there are treatments in the future that can be expected to halt myopia progression completely, all treatments are limited to reducing axial length growth rate and Rx progression. Consequently, to achieve the lowest final level of myopia at the end of the patient’s expected progression period, treatments must either be started early and/or must be highly effective.19 Only spectacle-based treatments could provide the necessary combination of efficacy with a near-zero risk profile to be used routinely in pre-myopia or low myopia.
Several novel spectacle approaches are in the late stages of development. The Hoya MiYoSmart20 lens with Defocus Incorporated Multiple Segments (DIMS) technology reports slowing axial eye growth on average by 60 percent and reducing myopia progression on average by 59 percent. It has a fascinating development tale. After some work establishing that dual-focus contact lenses were effective in controlling myopia, the researchers were brainstorming on how to achieve that level of success without the need to use a contact lens. In an inspiration to inventors everywhere, one of the DIMS lens inventors noticed how he could see through a mesh-type advertising film on a bus and thought that would be a great way to deliver dual-focus optics on a spectacle lens.
Essilor has just announced one year interim findings with the Stellest lens with Highly Aspheric Lenslet Technology or HALT. The Stellest lens has 11 rings of highly aspheric lenslets surrounding a clear annulus, a common feature of these novel lens designs. First-year results of over 60 percent control of myopia compared to control and stopping axial growth in 28 percent of children versus zero in the control group make this a promising lens to watch.
The SightGlass Vision DOT spectacle lens also features a clear zone surrounded by a myopia controlling annulus. SightGlass Vision has released one-year data on two spectacle lens designs studied in their Phase III CYPRESS clinical trial. On average Test 1 lens demonstrated a 74 percent reduction in cycloplegic spherical equivalent refraction and a 50 percent reduction in axial length progression. Test 2 lens showed a 59 percent reduction in cycloplegic spherical equivalent refraction and a 33 percent reduction in axial length progression. Its peripheral contrast-reduction proposed mechanism of action is unique among optical devices for myopia control.
Kubota Vision has announced early results from human trials of their concept of projecting myopia defocus into the eye showing favorable short-term axial length responses. Spectacle and contact lens applications are planned. Reopia, LLC, is in the early development stage of several spectacle-based novel myopia concepts.
Other novel myopia-controlling spectacles are also in development. A patent from Johnson & Johnson Vision describes a series of non-coaxial lenslets of high-plus power to distribute myopic defocus throughout regions of the retina. The company also has a fascinating electronic spectacle design, which provides a plus pulse at a frequency not detectible by the user to give a myopia suppression signal. A perusal of the patent literature finds many Johnson & Johnson Vision patents, contacts with high plus, pupil-independent designs, lenses that impose spherical aberration, lenses that emulate orthokeratology, contacts with non-coaxial optics and drug-eluting contacts, though without any products on the market. The company has received FDA Breakthrough Device designation for its yet unannounced myopia management contact lens, so it would appear products are on the way.
It is well known by now that bifocal and multifocal soft contact lenses control myopia progression quite effectively, with the first FDA approval for the MiSight 1 day lens coming last year and several other manufacturers, such as Visioneering Technologies, Paragon, SEED and mark’ennovy, with CE marks for myopia control. Bausch & Lomb just announced an exclusive agreement to develop novel EDOF myopia control contact lenses with BHVI.
Upward Trend in Myopia Treatment Patents
Inventors like to review the patent literature, but anyone interested in getting a glimpse of what is being proposed can start with the applications search at https://www.uspto.gov/ or https://patents.justia.com/. Table 1 shows how U.S. patent applications and patents granted in myopia treatment have changed dramatically from the early 2000s, when this author’s first patent was granted, to the current year.
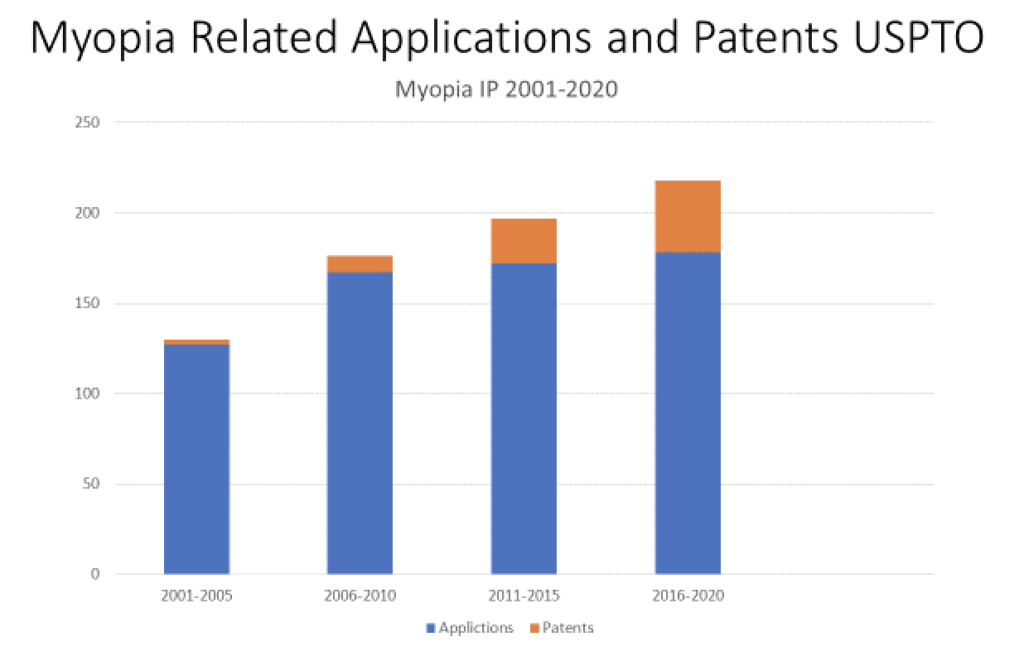
While 2020 should have been the year of vision and it tragically became anything but, it is still clearly an exciting time in the field of myopia management treatments. With all the current interventions and promising new treatments, myopia management is fast approaching the standard of care. Myopia is quaking in its boots.

Thomas Aller, OD, FBCLA, is a fellow of the British Contact Lens Association and an associate member of the International Society of Contact Lens Specialists. He is the 2018 recipient of the Award of Excellence from the Global Specialty Lens Symposium and the 2019 recipient of the Garland Clay Award from the American Academy of Optometry.
References:
- Holden BA, Fricke TR, Wilson DA, et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. May 2016;123(5):1036-42. doi:10.1016/j.ophtha.2016.01.006
- Huang J, Wen D, Wang Q, et al. Efficacy Comparison of 16 Interventions for Myopia Control in Children: A Network Meta-analysis. Ophthalmology. Apr 2016;123(4):697-708. doi:10.1016/j.ophtha.2015.11.010
- Aller T, Wildsoet C. Optical control of myopia has come of age: or has it? Optom Vis Sci. May 2013;90(5):e135-7. doi:10.1097/OPX.0b013e31828b47cf
- Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. Aug 2008;115(8):1279-85. doi:10.1016/j.ophtha.2007.12.019
- Xiong S, Sankaridurg P, Naduvilath T, et al. Time spent in outdoor activities in relation to myopia prevention and control: a meta-analysis and systematic review. Acta Ophthalmol. Sep 2017;95(6):551-566. doi:10.1111/aos.13403
- Smith EL, Hung LF, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. Jan 2012;53(1):421-8. doi:10.1167/iovs.11-8652
- Gawne TJ, Siegwart JT, Ward AH, Norton TT. The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Exp Eye Res. 02 2017;155:75-84. doi:10.1016/j.exer.2016.12.004
- Wang M, Schaeffel F, Jiang B, Feldkaemper M. Effects of Light of Different Spectral Composition on Refractive Development and Retinal Dopamine in Chicks. Invest Ophthalmol Vis Sci. 09 2018;59(11):4413-4424. doi:10.1167/iovs.18-23880
- Hung LF, Arumugam B, She Z, Ostrin L, Smith EL. Narrow-band, long-wavelength lighting promotes hyperopia and retards vision-induced myopia in infant rhesus monkeys. Exp Eye Res. 11 2018;176:147-160. doi:10.1016/j.exer.2018.07.004
- Chen Z, Niu L, Xue F, et al. Impact of pupil diameter on axial growth in orthokeratology. Optom Vis Sci. Nov 2012;89(11):1636-40. doi:10.1097/OPX.0b013e31826c1831
- Montés-Micó R, Charman WN. Pharmacological Strategies for Presbyopia Correction. J Refract Surg. Dec 2019;35(12):803-814. doi:10.3928/1081597X-20191010-04
- Yuda K, Uozato H, Hara N, et al. Training regimen involving cyclic induction of pupil constriction during far accommodation improves visual acuity in myopic children. Clin Ophthalmol. Apr 2010;4:251-60. doi:10.2147/opth.s9249
- Cooper J, Schulman E, Jamal N. Current status on the development and treatment of myopia. Optometry. May 2012;83(5):179-99.
- Trier K, Munk Ribel-Madsen S, Cui D, Brøgger Christensen S. Systemic 7-methylxanthine in retarding axial eye growth and myopia progression: a 36-month pilot study. J Ocul Biol Dis Infor. Dec 2008;1(2-4):85-93. doi:10.1007/s12177-008-9013-3
- Arumugam B, Hung L-F, Jong M, Ostrin L, Smith III E. Topically applied caffeine, a non-selective adenosine antagonist, alters emmetropizing responses in infant monkeys. Presented at: International Myopia Conference; 2017 Birmingham, UK.
- Cheng D, Woo GC, Drobe B, Schmid KL. Effect of bifocal and prismatic bifocal spectacles on myopia progression in children: three-year results of a randomized clinical trial. JAMA Ophthalmol. Mar 2014;132(3):258-64. doi:10.1001/jamaophthalmol.2013.7623
- Gwiazda J, Hyman L, Hussein M, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. Apr 2003;44(4):1492-500.
- Flitcroft DI, He M, Jonas JB, et al. IMI – Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Invest Ophthalmol Vis Sci. 02 2019;60(3):M20-M30. doi:10.1167/iovs.18-25957
- Aller TA. Clinical management of progressive myopia. Eye (Lond). Feb 2014;28(2):147-53. doi:10.1038/eye.2013.259
- Lam CSY, Tang WC, Tse DY, et al. Defocus Incorporated Multiple Segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomised clinical trial. Br J Ophthalmol. Mar 2020;104(3):363-368. doi:10.1136/bjophthalmol-2018-313739












