April 1, 2022
By Geetha Sravani, PhD Candidate, Brien Holden Vision Institute
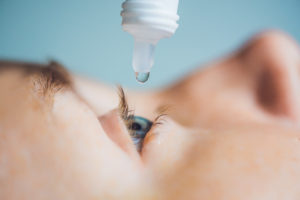
Myopia prevalence is high in many Asian countries. To delay the progression of myopia, a variety of strategies are in use, including: increased outdoor activity, less near work, peripheral defocusing lenses, orthokeratology contact lenses, and different concentrations of atropine.
Even though atropine is well known for its effectiveness in preventing myopia progression, there is currently no well-supported evidence to rank the clinical outcomes of various atropine concentrations. In this regard, Ahnul et al. (2021) conducted a systematic review and network meta-analysis (NMA) to compare the efficacy and safety of eight atropine concentrations (0.1 – 0.01%) for myopia control. They selected randomized control trials (RCTs) involving atropine treatment of at least one-year duration for myopia control in children following a detailed search.
In total, 16 RCTs were selected and enabled 30 pairwise comparisons. The primary outcomes were refraction (D/year) and axial length (AXL) (mm/year) with mean annual changes. According to the NMA rankings, atropine concentrations of 1%, 0.5%, and 0.05% were the most beneficial for myopia control treatment for both primary outcomes: 1% atropine (mean differences compared with control: refraction, 0.81 [95% confidence interval (CI), 0.58 -1.04]; AXL, -0.35 [-0.46 to -0.25]); 0.5% atropine (refraction, 0.70 [95% CI, 0.40 – 1.00]; AXL, -0.23 [-0.38 to -0.07]); 0.05% atropine (refraction, 0.62 [95% CI, 0.17 – 1.07]; AXL, -0.25 [-0.44 to -0.06]). A negative MD in AXL indicates that the first intervention was better, i.e., less axial elongation. In terms of Relative Risk (RR), 0.05% atropine concentration showed the lowest RR for overall myopia progression (RR, 0.39; 95% CI, 0.27 – 0.57), followed by 1% (RR, 0.43; 95% CI, 0.33 – 0.56). When the atropine concentration was increased, the probability of adverse effects rose, but this trend was not seen for distance BCVA. It was concluded that even though the pupil size and accommodation amplitude were dose-related, the ranking probability for efficacy was not directly proportional to dose.
Abstract
Efficacy and Safety of 8 Atropine Concentrations for Myopia Control in Children: A Network Meta-Analysis
Ahnul Ha, Seong Joon Kim, Sung Ryul Shim, Young Kook Kim, Jae Ho Jung
Topic: Comparative efficacy and safety of different concentrations of atropine for myopia control.
Clinical Relevance: Atropine is known to be an effective intervention to delay myopia progression. Nonetheless, no well-supported evidence exists yet to rank the clinical outcomes of various concentrations of atropine.
Methods: We searched PubMed, EMBASE, Cochrane Central Register of Controlled Trials, the World Health Organization International Clinical Trials Registry Platform, and ClinicalTrials.gov on April 14, 2021. We selected studies involving atropine treatment of at least 1 year’s duration for myopia control in children. We performed a network meta-analysis (NMA) of randomized controlled trials (RCTs) and compared 8 atropine concentrations (1% to 0.01%). We ranked the atropine concentrations for the corresponding outcomes by P score (estimate of the probability of being the best treatment). Our primary outcomes were mean annual changes in refraction (dioptres/year) and axial length (AXL; millimeters/year). We extracted data on the proportion of eyes showing myopia progression and safety outcomes (photopic and mesopic pupil diameter, accommodation amplitude, distance, and near best-corrected visual acuity [BCVA]).
Results: Thirty pairwise comparisons from 16 RCTs (3,272 participants) were obtained. Our NMA ranked the 1%, 0.5%, and 0.05% atropine concentrations as the 3 most beneficial for myopia control, as assessed for both primary outcomes: 1% atropine (mean differences compared with control: refraction, 0.81 [95% confidence interval (CI), 0.58e1.04]; AXL, e0.35 [e0.46 to e0.25]); 0.5% atropine (mean differences compared with control: refraction, 0.70 [95% CI, 0.40e1.00]; AXL, e0.23 [e0.38 to e0.07]); 0.05%atropine (mean differences compared with control: refraction, 0.62 [95% CI, 0.17e1.07]; AXL, e0.25 [e0.44 to e0.06]). In terms of myopia control as assessed by relative risk (RR) for overall myopia progression, 0.05% was ranked as the most beneficial concentration (RR, 0.39 [95% CI, 0.27e0.57]). The risk for adverse effects tended to rise as the atropine concentration was increased, although this tendency was not evident for distance BCVA. No valid network was formed for near BCVA.
Discussion: The ranking probability for efficacy was not proportional to dose (i.e., 0.05% atropine was comparable with that of high-dose atropine [1% and 0.5%]), although those for pupil size and accommodation amplitude were dose-related.
Ha, A., Kim, S. J., Shim, S. R., Kim, Y. K., & Jung, J. H. (2022). Efficacy and safety of 8 atropine concentrations for myopia control in children: a network meta-analysis. Ophthalmology, 129(3), 322-333.
DOI: 10.1016/j.ophtha.2021.10.016
 |
Geetha Sravani is a PhD candidate at the Brien Holden Vision Institute, supervised by Prof. Padmaja Sankaridurg and Arthur Back. Her research focuses on the role of pupillary responses in myopia.
|