September 15, 2021
By Andrei V. Tkatchenko, MD, PhD, and Tatiana V. Tkatchenko, MD
The development of effective and safe drugs for myopia control is not a distant future.
Dioptragen Therapeutics is a start-up drug development company that targets myopia. Dioptragen uses its proprietary pharmacogenomics platform to identify and test novel drug compounds that suppress myopia. On December 15, 2020, Columbia University granted us an exclusive license that allowed Dioptragen to commercialize a pharmacogenomics platform for drug development. The license also grants Dioptragen an exclusive right to develop anti-myopia eye drops and other ophthalmic formulations based on the drug compounds identified by the pharmacogenomics platform.
Both environmental and genetic factors control the development of myopia. Human population studies suggest that the leading environmental factors causing human myopia are near work and reading. The optical blur associated with near work is believed to be the signal that drives excessive eye growth and causes myopia.1,2 The association between optical defocus and myopia is supported by numerous animal studies. These studies have found that degradation of visual input using either diffusers or negative lenses causes excessive eye growth and myopia in species as diverse as fish, chickens, tree shrews, monkeys, guinea pigs, and mice.3
Although the increase in the prevalence of myopia in recent decades is primarily attributed to the rapidly increasing exposure of young children to near work, both environmental and genetic factors have been shown to contribute to myopia development.4 Moreover, a recent study has demonstrated the existence of genes that modulate the impact of myopiagenic environmental factors on refractive eye development.5 Further support for gene-environment interaction in myopia development comes from gene-expression-profiling studies, which have uncovered that the development of myopia is accompanied by large-scale changes in gene expression in the eye. This suggests that near work activates molecular signaling pathways in the eye that stimulate excessive eye growth, leading to myopia development.6 Several studies have revealed that the eye responds to local changes in optical defocus with local changes in growth rate. This suggests that information about optical defocus is summed up across the entire surface of the retina, which creates an integrated signal that regulates the growth of the eye.7,8 This demonstrates that the signaling cascade regulating refractive eye development is located within the eye itself and does not require feedback from the brain.
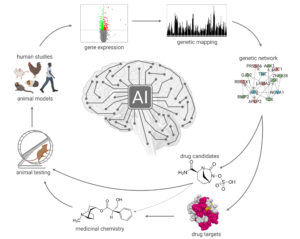
Figure 1. The pharmacogenomics platform for anti-myopia drug development takes advantage of the latest developments in systems genetics and pharmacogenomics. Systems genetics provides information about gene regulatory networks underlying refractive eye development. The information about these gene regulatory networks is processed using cutting-edge bioinformatics to identify potential drug targets and drug candidates.
Currently approved treatment options for myopia are mostly limited to optical correction using spectacles or contact lenses. Optical correction using single-vision corrective lenses, which is the most widely used treatment option for myopia, does not stop the progression of myopia and does not prevent the blinding pathological complications associated with the disease.9,10 Several experimental optics-based clinical interventions that are meant to slow myopia progression, such as spectacles with bifocal lenses, multifocal contact lenses, and orthokeratology lenses have shown some promise. However, these treatment options have relatively low efficacy.11
Pharmacogenomics Platform for Anti-Myopia Drug Development
Dioptragen has developed a pharmacogenomics platform to identify drugs capable of suppressing myopia development (Figure 1).12 We used systems genetics to identify the genes, genetic networks, and signaling pathways underlying refractive eye development and myopia. This systems genetics approach involved identifying genes differentially expressed in animals’ eyes with experimentally induced myopia using whole-genome gene expression profiling and the identification of genes linked to myopia in mice and humans using genetic mapping and gene-based genome-wide association studies. We characterized the gene regulatory networks underlying baseline refractive eye development and visually guided eye emmetropization. We also found that the signaling pathways underlying the eye’s response to positive optical defocus (which suppresses myopia) and negative optical defocus (which promotes myopia development) propagate via two largely distinct signaling cascades. We extended these observations to several vertebrate species and demonstrated that the signaling cascades underlying myopia development are highly evolutionarily conserved across vertebrate species, including humans. We then used our vast myopia-associated gene dataset generated during these studies to reconstruct the gene regulatory networks that control myopia development. The information about these gene networks was then used to develop a proprietary pharmacogenomics platform for anti-myopia drug development. This platform can identify drug compounds that can suppress signaling pathways that promote myopia development and stimulate the pathways that inhibit myopia development.
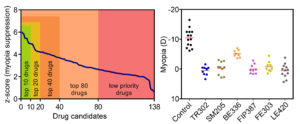
Figure 2. The pharmacogenomics platform for anti-myopia drug development has identified over 130 drug candidates (left panel). The top six drug candidates showed high efficacy in initial pre-clinical studies; drugs were tested using a mouse model of myopia (right panel).
Dioptragen’s Drug Pipeline
Dioptragen has identified over 130 drug compounds with anti-myopic potential (Figure 2). These drug compounds were divided into several priority categories based on their predicted potential to suppress myopia and known or predicted side effects. Atropine was picked up by the pharmacogenomics platform as one of the anti-myopic compounds and was placed in the top 20 group. Dioptragen is currently using animal models to conduct systematic pre-clinical studies of the candidate drugs identified by the pharmacogenomics platform. The company has completed initial pre-clinical studies of the top six drugs, which belong to six novel drug classes not previously implicated in myopia control. These studies have demonstrated their high efficacy and good safety profiles. While five of these drugs are still in pre-clinical stages of development, one of the drugs (LE420) is ready to advance to a phase II clinical trial.

Andrei V. Tkatchenko, MD, PhD, is a molecular biologist who has been studying the molecular signaling underlying myopia development for more than 20 years. He holds an MD in medical biology and MS in molecular biology from the Russian National Research Medical University. He also holds a PhD in molecular biology from the Engelhardt Institute of Molecular Biology of the Russian Academy of Sciences and has received training in visual neuroscience at Harvard University. Since 2014, he has been working as an Associate Professor of Ophthalmic Sciences at Columbia University in New York.

Tatiana V. Tkatchenko, MD, is a pediatric geneticist who has been working in myopia research for more than 15 years. She received an MD in pediatrics from the Russian National Research Medical University and has received training in pediatric genetics from the Université Montpellier II in France. She has also received training in molecular genetics at Harvard University. She has been working as an Associate Research Scientist at Columbia University in New York since 2014.
References
- Charman WN. Near vision, lags of accommodation and myopia. Ophthalmic Physiol Opt 1999;19:126-133.
- Gwiazda JE, Hyman L, Norton TT, et al. Accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. Invest Ophthalmol Vis Sci 2004;45:2143-2151.
- Troilo D, Smith EL, 3rd, Nickla DL, et al. IMI – Report on Experimental Models of Emmetropization and Myopia. Invest Ophthalmol Vis Sci 2019;60:M31-M88.
- Wojciechowski R. Nature and nurture: the complex genetics of myopia and refractive error. Clin Genet 2011;79:301-320.
- Tkatchenko AV, Tkatchenko TV, Guggenheim JA, et al. APLP2 Regulates Refractive Error and Myopia Development in Mice and Humans. PLoS Genet 2015;11:e1005432.
- Summers JA, Schaeffel F, Marcos S, Wu H, Tkatchenko AV. Functional integration of eye tissues and refractive eye development: Mechanisms and pathways. Exp Eye Res 2021;108693.
- Smith EL, 3rd. Prentice Award Lecture 2010: A case for peripheral optical treatment strategies for myopia. Optom Vis Sci 2011;88:1029-1044.
- Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Curr Eye Res 1987;6:993-999.
- Ong E, Grice K, Held R, Thorn F, Gwiazda J. Effects of spectacle intervention on the progression of myopia in children. Optom Vis Sci 1999;76:363-369.
- Walline JJ, Jones LA, Sinnott L, et al. A randomized trial of the effect of soft contact lenses on myopia progression in children. Invest Ophthalmol Vis Sci 2008;49:4702-4706.
- Wildsoet CF, Chia A, Cho P, et al. IMI – International Myopia Institute: Interventions for Controlling Myopia Onset and Progression Report. Invest Ophthalmol Vis Sci 2019;60:M106-M131.
- Tkatchenko TV, Tkatchenko AV. Pharmacogenomic approach to antimyopia drug development: pathways lead the way. Trends Pharmacol Sci 2019;40:834-853.













