February 15, 2023
By Michael Lipson, OD, FAAO, FSLS; Jackson Lau, OD, FAAO, FSLS; and Craig Norman, FCLSA
Modern orthokeratology (OrthoK) is over two decades old and has shown wide-ranging patient acceptance and significant growth around the globe. Today, over 20 manufacturer-specific designs are being taught in all optometric schools. OrthoK is also gaining traction within ophthalmology as an option for myopia management for young children and adults alike.
Additionally, among practitioners actively fitting orthokeratology, it’s been accepted that the use of baseline and follow-up visit corneal topography is the standard of care, regardless of the fitting method used to determine the initial lens design.
Numerous high-quality corneal topographers and tomographers are available today — Medmont, Oculus Keratograph, Pentacam, Zeiss Atlas, Topcon CA-800, and more. [This article references the Medmont due to its annotation capabilities.]
Using the Corneal Topographer
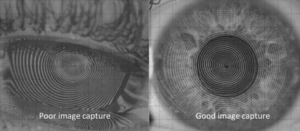
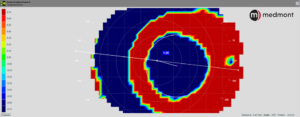
Make certain there is a quality image capture. We’ve all heard the phrase “garbage in, garbage out,” referring to flawed input data producing flawed output. This is true for capturing corneal topography images, as well. (Figure 1) If there is excessive tearing or the corneal surface begins to dry, the Placido rings will be unclear. In addition, if the lid aperture is small, it will limit the area of corneal mapping. These are especially important for the baseline measurements as they will be used as a reference throughout the course of OrthoK wear.
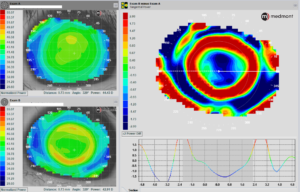
Use the proper scale for a better view of the acquired map. During a topography lecture, did you ever wonder why the clarity and definition of the maps discussed are so much better than yours? Although it could be because of poor captures, it may be due to the scale. The scale can be significantly magnified, resulting in clearer images. (Figure 2) This is especially noticeable when looking for the bullseye pattern or treatment zone size and location.
Choose the type of map based on what you’re analyzing — axial, tangential, or elevation. Each map has a specific function. Axial maps are suitable for baseline measurements and to demonstrate the surface power of the cornea. Tangential maps show a more accurate picture of corneal shape and thus are ideal for evaluating the size and location of the treatment zone after OrthoK. Elevation maps are helpful in analyzing the shape differences between different OrthoK meridians, which aid in selecting a toric vs. a spherical OrthoK design.
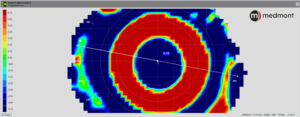
Never use a post-treatment map alone; instead, use a difference (subtractive map) for comparison. (Figure 3)
Since it’s routine that OrthoK patients don’t return for follow-up care wearing their lenses, the only method to judge the impact of the reverse geometry design is by comparing the initial baseline topography maps to those taken on the day of the follow-up visit. This comparison provides a snapshot of the central changes made to the corneal profile, what is termed the treatment zone. (Figure 4)
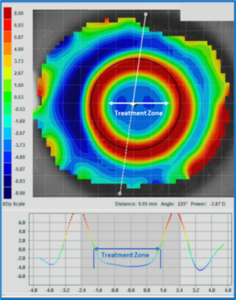
Figure 4 – The location of the topography treatment zone defined as the point where the OrthoK-induced corneal changes (flattening) representing no change from baseline zero is demonstrated here as the middle of the green color.
Our focus is on accurately describing the technique to determine treatment zone decentration (TZDec) and not to imply how these findings may impact refractive correction or axial elongation with OrthoK.
Quantification of Decentration in OrthoK
The position of the treatment zone may have clinical significance in assessing aberrations and for the efficacy of slowing axial elongation. Clinical evaluation of lens positioning during OrthoK can be performed under various conditions and with different techniques. First, with the lens on the cornea, the lens position in the open eye condition can be evaluated at the slit lamp.
This process has limitations due to interaction with the upper eyelid before, during, and after blinking. In addition, it is difficult to quantify the distance from the center of the lens to the geometrical center of the cornea. Clinicians have historically recorded lens position as “well-centered,” slightly, moderately, or significantly decentered, followed by the direction (i.e., nasal, inferior temporal, etc.). Even the most experienced clinician may not be able to assess the deviation from the center within a range of 0.50 – 1.00 mm.
A variation of this technique is to estimate the distance from the edge of the lens to the limbus in various meridians to estimate the deviation of the lens from the geometrical center of the cornea.
A second method of determining OrthoK lens decentration uses post-treatment topography. Post-treatment topography maps the corneal changes induced by overnight wear of an OrthoK lens. The location of these changes can then be assessed to determine TZDec. Using this technique to measure the TZDec allows for a precise way to evaluate the position of an OrthoK lens in the closed-eye condition.
This technique has two significant advantages over the slit lamp method described above:
1) It assesses induced topographical changes of the cornea after wearing an OrthoK lens while sleeping. This “closed eye” lens position determines what the eye and the patient experience during waking hours without the lens on the eye.
2) It is far more precise.
The technique described here uses software within the Medmont topography system. It employs measurements with drawing tools followed by a manual vector to measure the distance and direction from the geometrical center of the cornea to the center of the treatment zone.
From a consensus of the authors, these measurements can be made in the range of 0.1 mm of distance and 1º of the exact meridian. To determine the precise magnitude of TZDec, the position of the treatment zone must be defined.
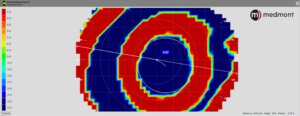
Looking at the post-treatment tangential difference map, a circle tool is started on the left side of the map in the flat meridian (generally horizontal) at a point in the center of the green color surrounding the central flattened (blue) area (representing no curvature change from baseline).
The circle is then pulled with the cursor to the corresponding green point on the right of the map. The created white circle is then moved up or down to create an equal vertical distance from the superior and inferior margins of the green area. The center of that “created” circle is automatically displayed as a cross hair.
Subsequently, a measuring tool is used to mark the distance from the geometric center of the cornea to the center of the circle. The software automatically displays that distance (in mm to two decimal points) and the axis of orientation. For example, figure 5 below shows the decentration as 0.48mm @ 187°.
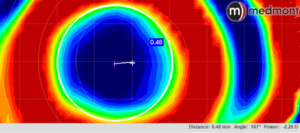
Figure 5 – A magnified tangential difference map of a post-OrthoK patient with a white circle created to identify the position of the treatment zone. The center of the circle is automatically displayed. The distance and meridian of the crosshair from the geometric center of the cornea (shown in the lower right of the figure) are then measured with a ruler contained in the software. The result is a precise assessment of treatment zone decentration.
A recent study by Guo et al. compared results using manual vs. software-based techniques.1 They reported manual and software-based measurements were not identical and could not be used interchangeably. The software-based results may be less time consuming, more objective, and can be performed by less-experienced practitioners. This study could not determine which method (manual or software-based) was more accurate in measuring treatment zone parameters.
Evaluating and quantifying decentration in OrthoK with post-treatment topography has been reported in previous publications over the last 15 years.1–4 They were able to report on mean decentration with various lenses and techniques, but no clinical implications were made.4
Hiraoka et al. published a study in 2009 that described their manual process of evaluating OrthoK decentration in detail.2 They used a specialized data analysis program to create a circle or ellipse of the treatment zone and then determined the magnitude and direction of the center of the shape from the center of the entrance pupil.
A recent study1 used new assessment techniques but applied them to subjects from previous studies published in 2012 (ROMIO),5 2013 (TO-SEE),6 and 2019 (OKIC).7 They retrospectively evaluated decentration among subjects classified as slow progressors and fast progressors.
From these studies, three features become apparent:
1) Measurement of TZDec (quantity and direction) can be very precise.
2) The manual techniques can be cumbersome and time consuming, while the software-based techniques are more straightforward and quicker.
3) The results of these measurements may have significant clinical implications on OrthoK fitting and the efficacy of OrthoK to slow axial elongation.
Why Is This Important for the Average OrthoK Practitioner?
Modern refractive surgery utilizes precise instrumentation to provide patients with the best possible outcomes. Wavefront and topography-guided techniques have become popularized, allowing surgeons to carefully shape the contour of the eye while minimizing unwanted higher-order aberrations. As OrthoK is an alternative to refractive surgery, technology exists to match the precision offered by our surgical colleagues.
The efficacy of OrthoK may improve by precise measurement of TZDec. The benefits may include the following:
- Improved communication with laboratory consultants
- Enhanced OrthoK fitting techniques for improvement in daily vision quality and corneal health
- Collection of empirical data to optimize myopia management
Improve Communication with Laboratory Manufacturers
Obtaining objective measurements of OrthoK decentration may allow laboratory manufacturers to guide lens modifications more precisely for improved patient outcomes. Our current estimation methods of assessing OrthoK sodium fluorescein patterns and lens centration on-eye in the upright, open-eye position provide an inaccurate assessment of the actual closed-eye positioning.
However, estimating the topographic patterns without quantitative analysis can just as easily lead to bias and inaccurate data. Quantifying OrthoK decentration can significantly inform our design processes, such as determining the need for a toric design or choosing critical parameters such as lens diameter and alignment curve radii.
While taking the measurements is the first step, categorizing the magnitude of decentration can provide an important context for successful long-term management.
For many years, refractive surgeons have categorized ablation decentration from the pupil center as seen in Table 1.8 More recently, Guo et al.9 and Sun et al.10 adapted these similar categories to define OrthoK TZDec, providing practitioners a guideline to reference when managing patients. More consistency can be created among practitioners of varying skill levels and experience by universally adopting a standard.
Figure 6 – Examples of mild (A), moderate (B), and significant (C) treatment zone decentration
Improve Vision Quality
OrthoK decentration has been reported to cause visual discomfort, aberrations, and distortions.2,11,12 By optimizing centration, patients experience superior vision quality, which can be critical for adult patients with visual demands in low-light situations such as night driving.
Though prevailing clinical wisdom and anecdotal evidence suggest children may be more adaptable to the negative visual effects of OrthoK decentration, the visual demands of children are increasing; practitioners should aim to balance visual quality with the goals of myopia management.
Corneal Health
It has been suggested that excessive rigid-lens decentration may cause corneal indentation, epithelial staining, lens adherence to the cornea, corneal warpage, and corneal hypoxia.12–14 More recently, a case report found a possible association between microcystic corneal edema and wear of decentered OrthoK lenses.9 Though few studies evaluate the long-term corneal health impact of OrthoK decentration, eye care practitioners should make efforts to optimize lens centration.
Myopia Management
Many researchers have studied the potential risks and benefits of OrthoK decentration regarding the slowing of axial elongation and myopia progression.
A recent study by Sun et al.10 found no significant difference in axial length change in patients with different magnitudes of OrthoK decentration. The authors recommended properly centered treatments for safety and visual quality. In contrast, other studies found that axial elongation was inversely correlated with the magnitude of OrthoK TZDec.12,15 Though there is currently no consensus, the ability to incorporate future emerging scientific evidence into clinical practice relies on precise measurement of OrthoK TZDec now.
Balancing myopia management with myopia correction will be key for our pediatric patients. Future directions for OrthoK advancements may involve optimal lens centration for corneal health, combined with decentered optic zones of varying degrees to create patient-specific treatment zones. The peer-reviewed literature will continue to guide these developments.
What Can We Expect Topography Companies To Do?
Often, we take for granted that current glaucoma instrumentation uses sophisticated algorithms developed over multiple decades to help practitioners determine the progression of the disease. Similarly, precise measurements of post-treatment topography may help provide a more detailed analysis to advance OrthoK and myopia management algorithms.
For example, many publications utilized custom software for treatment zone analysis.2,15–17 Deep learning and artificial intelligence continue to garner tremendous interest in all fields, including health care. Tang et al.18 recently utilized automated deep learning methods to define OrthoK treatment zones using Pentacam software and found they could do so accurately and efficiently. Reducing the time and resources needed to assess topographical patterns critically may significantly and positively impact modern clinical practice.
Another advantage of automating this process is reducing observer bias and improving repeatability issues inherent to manual methods. As noted above, current fixation techniques are largely a manual process of trial and error and are highly dependent on patient understanding and cooperation.
Next-generation topography technology can aid practitioners by providing improved fixation tools, similar to gaze-tracking functionality in visual fields or manually adjustable fixation targets in fundus cameras. In addition, standardizing the OrthoK industry such that topographies are based on geometrically centered maps may improve OrthoK outcomes. Such tools would allow for more accurate and consistent decentration measurements, as patients would maintain the same fixation points during baseline and every follow-up visit.
What Can Practitioners Do Before Next-Gen Topographers Are Available?
To the best of our abilities, terms such as “well-centered” and “adequate” should be replaced by quantitative values. As with all other systems, it is important to follow a standard protocol; consistency is key to taking accurate measurements.
Eventually, future research will determine how much decentration is acceptable or necessary to optimize myopia management. Until then, it would be prudent to start gathering this data using measurement techniques described in this paper to analyze trends. By starting to track that data now, patients will be better served in the long term.
 |
Dr. Michael Lipson is recently retired from his position as an optometrist/associate professor at University of Michigan, department of Ophthalmology and Visual Science. His clinical practice involves specialty contact lenses: OrthoK, keratoconus, post-corneal transplant, post-refractive surgery, and severe dry eye patients. He has published peer-reviewed clinical research studies on OrthoK, vision-related quality of life, myopia management and new lens designs. He lectures nationally and internationally on those same topics. Dr. Lipson developed a validated questionnaire to assess vision-related quality of life for all types of vision correction, including OrthoK. He has authored chapters in textbooks on OrthoK, scleral lenses, and general contact lens topics. Dr. Lipson is the author of the book Contemporary OrthoKeratology. He also is a reviewer for a number of highly-respected peer-reviewed journals in the ophthalmic community. He is a consultant to the specialty contact lens industry emphasizing OrthoK education. He is on the GPLI Advisory Board, served as Vice-President of the Scleral Lens Education Society, and served on the Scleral Lens Education Society Board for many years. |
 |
Dr. Jackson Lau is the Clinical and Scientific Affairs Manager at Euclid Vision Corporation. He obtained his Doctor of Optometry from the UC Berkeley School of Optometry. After completing a residency in Cornea and Contact Lenses at the Illinois College of Optometry, he joined a high-volume specialty contact lens and myopia management practice in Sunnyvale, CA. Dr. Lau has conducted research on dry eyes and scleral contact lenses and has lectured on topics of corneal disease, myopia management, and specialty contact lenses. He earned distinction as a Fellow of the Scleral Lens Education Society and is a Fellow of the American Academy of Optometry. Dr. Lau directed the clinical externship program at Silicon Valley Eye Physicians and served as a sub-investigator for multiple contact lens and ophthalmic drug trials. Since joining Euclid, Dr. Lau focuses on academic and professional relations. He continues to practice in the Silicon Valley and serves as an adjunct clinical faculty for the Illinois College of Optometry and New England College of Optometry. |
 |
Craig Norman is an adjunct faculty member at the Michigan College of Optometry’s Vision Research Institute. He is co-curator of the Contact Lens Museum. He has received speaking honoraria or consulting fees from ABB Optical Group, Bausch Medical, Contamac, Euclid Systems, GPLI, Lentechs, Oculus USA, Precilens, and Valley Contax. |
References
- Guo B, Wu H, Cheung SW, Cho P. Manual and software‐based measurements of treatment zone parameters and characteristics in children with slow and fast axial elongation in orthokeratology. Ophthalmic Physiol Opt. Published online April 2, 2022:opo.12981. doi:10.1111/opo.12981
- Hiraoka T, Mihashi T, Okamoto C, Okamoto F, Hirohara Y, Oshika T. Influence of induced decentered orthokeratology lens on ocular higher-order wavefront aberrations and contrast sensitivity function. J Cataract Refract Surg. 2009;35(11):1918-1926. doi:10.1016/j.jcrs.2009.06.018
- Mei Y, Tang Z, Li Z, Yang X. Repeatability and Reproducibility of Quantitative Corneal Shape Analysis after Orthokeratology Treatment Using Image-Pro Plus Software. J Ophthalmol. 2016;2016:1732476. doi:10.1155/2016/1732476
- Yang X, Zhong X, Gong X, Zeng J. Topographical evaluation of the decentration of orthokeratology lenses. Yan Ke Xue Bao. 2005;21(3):132-135, 195.
- Cho P, Cheung SW. Retardation of Myopia in Orthokeratology (ROMIO) Study: A 2-Year Randomized Clinical Trial. Investig Opthalmology Vis Sci. 2012;53(11):7077. doi:10.1167/iovs.12-10565
- Chen C, Cheung SW, Cho P. Myopia Control Using Toric Orthokeratology (TO-SEE Study). Investig Opthalmology Vis Sci. 2013;54(10):6510. doi:10.1167/iovs.13-12527
- Lau JK, Wan K, Cho P. Orthokeratology lenses with increased compression factor (OKIC): A 2-year longitudinal clinical trial for myopia control. Contact Lens Anterior Eye J Br Contact Lens Assoc. Published online August 19, 2022:101745. doi:10.1016/j.clae.2022.101745
- Tsai YY, Lin JM. Ablation centration after active eye-tracker-assisted photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refract Surg. 2000;26(1):28-34. doi:10.1016/S0886-3350(99)00328-4
- Guo B, Cho P, Efron N. Microcystic corneal oedema associated with over-wear of decentred orthokeratology lenses during COVID-19 lockdown. Clin Exp Optom. 2021;0(0):1-5. doi:10.1080/08164622.2021.1896944
- Sun L, Li ZX, Chen Y, He ZQ, Song HX. The effect of orthokeratology treatment zone decentration on myopia progression. BMC Ophthalmol. 2022;22(1):76. doi:10.1186/s12886-022-02310-4
- Lu F, Simpson T, Sorbara L, Fonn D. The relationship between the treatment zone diameter and visual, optical and subjective performance in Corneal Refractive Therapy lens wearers. Ophthalmic Physiol Opt J Br Coll Ophthalmic Opt Optom. 2007;27(6):568-578. doi:10.1111/j.1475-1313.2007.00520.x
- Chen R, Chen Y, Lipson M, et al. The Effect of Treatment Zone Decentration on Myopic Progression during Orthokeratology. Curr Eye Res. 2020;45(5):645-651. doi:10.1080/02713683.2019.1673438
- Lin W, Gu T, Bi H, Du B, Zhang B, Wei R. The treatment zone decentration and corneal refractive profile changes in children undergoing orthokeratology treatment. BMC Ophthalmol. 2022;22(1):177. doi:10.1186/s12886-022-02396-w
- Wilson SE, Lin DT, Klyce SD, Reidy JJ, Insler MS. Rigid contact lens decentration: a risk factor for corneal warpage. CLAO J Off Publ Contact Lens Assoc Ophthalmol Inc. 1990;16(3):177-182.
- Lin W, Li N, Gu T, et al. The treatment zone size and its decentration influence axial elongation in children with orthokeratology treatment. BMC Ophthalmol. 2021;21(1):362. doi:10.1186/s12886-021-02123-x
- Maseedupally VK, Gifford P, Lum E, et al. Treatment Zone Decentration During Orthokeratology on Eyes with Corneal Toricity. Optom Vis Sci. 2016;93(9):1101-1111. doi:10.1097/OPX.0000000000000896
- Gu T, Gong B, Lu D, et al. Influence of Corneal Topographic Parameters in the Decentration of Orthokeratology. Eye Contact Lens Sci Clin Pract. 2019;45(6):372-376. doi:10.1097/ICL.0000000000000580
- Tang Y, Chen Z, Wang W, et al. A Deep Learning-Based Framework for Accurate Evaluation of Corneal Treatment Zone After Orthokeratology. Transl Vis Sci Technol. 2021;10(14):21. doi:10.1167/tvst.10.14.21