sponsored content
October 1, 2020
By Noel A. Brennan, MScOptom, PhD, FAAO
Fellow, Johnson & Johnson Vision
In recent years you have almost certainly heard people throw around percentages to describe and compare the treatment effects of different myopia control therapies. Well, it’s time to throw away those percentages. A careful reading of the literature, critical analysis of data, and adherence to evidence-based principles has led us to identify the default optimal way to express treatment efficacy observed with myopia control – Cumulative Absolute Reduction in axial Elongation (CARE).1 It’s actually not new. It’s already presented in any half-decent clinical trial report, and it is simple and intuitive. Our contribution is limited to identifying this as the best metric and then putting an acronym to it to make it more prominent and accessible. Let’s break down why CARE should be adopted as the gold standard method for describing myopia control treatment efficacy.
The ‘C’ in CARE is for ‘Cumulative’ because annual treatment efficacy does not capture decreasing effect size over time. Treatments can produce a major reduction in axial elongation (even shrinkage) in the first few months, but the effect diminishes with time on both an absolute and percentage basis.1 Consequently, a treatment might show, say, a mean of 50 percent efficacy in year one but only 6 percent in the fifth year and over the five years in total only 30 percent. These are real data derived from Hiraoka et al.4 The best representation is to provide the cumulative treatment effect and study duration. From an evidence-based perspective, we can confidently say that a given CARE has been proven, but anything beyond the demonstrated effect or duration is speculative.
The ‘A’ in CARE is for ‘Absolute,’ indicating that effect size for any given treatment trends toward a constant absolute, rather than relative, value across a range of important demographic parameters such as age, progression rate, baseline myopia, and ethnicity.1 To put this in further context, two studies evaluating exactly the same treatment could both demonstrate a CARE of 0.15mm over one year but report dramatic differences in percentage treatment effect. The 0.15mm CARE might amount to 100 percent efficacy in an older Caucasian cohort, whereas a younger Asian cohort might show 25 percent efficacy over that time. In this context, CARE provides a more independent and consistent representation of treatment efficacy, demonstrating the merit and appeal of this metric.
The ‘RE’ in CARE comes from ‘Reduction in axial Elongation.’ Axial elongation is preferred over refractive error to describe progression because it is more accurate1 and shows a stronger association with eye disease and vision loss later in life.2,3 Optical biometry is well over three times more repeatable than cycloplegic autorefraction and is the only way to accurately assess progression with orthokeratology and atropine treatments.1 Indeed, the repeatability of refractive error measurements is sufficiently poor that the noise in the system is of the same order of magnitude as the signal we are measuring. Axial length can also be measured accurately without cycloplegia, reducing the potential burden associated with more frequent measurements.
In addition to providing a better representation of the treatment effect across treatments or studies, CARE will also offer a better example of the potential treatment effect for an individual patient. Percentage results are derived from group averages. For a likely fast-progressing patient, the expected reduction in progression will be better expressed by CARE, but we should note that it will predict a substantially smaller treatment effect than percentage estimates advise. Since we cannot actually measure treatment effect in practice (we don’t have control data for individual children), CARE, as derived from clinical studies, is our best guide from the available evidence to date for estimating the efficacy that we are actually achieving. Of course, some normative data for annual axial elongation would help – we’re working on that!
Figure: Some representative CARE values for different treatments. Years of follow-up are indicated above each bar. An estimate of the dioptric effect can be obtained by multiplying CARE by 2.1. (OK: orthokeratology, A1.0 percent: 1.0 percent atropine drops, A0.05 percent: 0.05 percent atropine drops, SMCLs: soft multifocal contact lenses, Spec: spectacles).
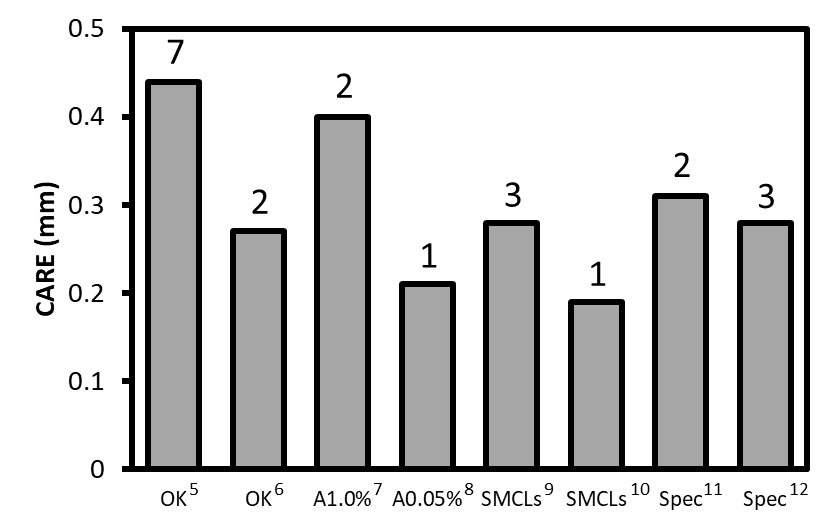

Noel A. Brennan, MScOptom, PhD, FAAO, is Clinical Research Fellow at Johnson & Johnson Vision Care, where he has been for the past nine years. Prior to that he co-directed a privately owned research consulting company and, before that, was a professor at the University of Melbourne. Under a Senior Fulbright Scholarship, Dr. Brennan was also visiting scholar in the Department of Surgery at Stanford University. He was recently named one of the 30 most influential people in the contact lens field over the past 30 years in Contact Lens Spectrum.
References:
- Brennan NA, Toubouti Y, Cheng X, Bullimore MA. Efficacy in myopia control. Prog Retin Eye Res 2020; in press.
- Gao LQ, Liu W, Liang YB et al. Prevalence and characteristics of myopic retinopathy in a rural Chinese adult population: the Handan eye study. Arch Ophthalmol 2011; 129:1199-1204.
- Tideman JW, Snabel MC, Tedja MS et al. Association of axial length with risk of uncorrectable visual impairment for Europeans with myopia. JAMA Ophthalmol 2016;134:1355-1363.
- Hiraoka T, Kakita T, Okamoto F et al. Long-term effect of overnight orthokeratology on axial length elongation in childhood myopia: a 5-year follow-up study. Invest Ophthalmol Vis Sci 2012;53:3913-3919.
- Santodomingo-Rubido J, Villa-Collar C, Gilmartin B et al. Long-term Efficacy of Orthokeratology Contact Lens Wear in Controlling the Progression of Childhood Myopia. Curr Eye Res 2017;42:713-720.
- Cho P, Cheung SW. Retardation of myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci 2012;53:7077-7085.
- Chua WH, Balakrishnan V, Chan YH et al. Atropine for the treatment of childhood myopia. Ophthalmol 2006;113:2285-2291.
- Yam JC, Jiang Y, Tang SM et al. Low-Concentration Atropine for Myopia Progression (LAMP) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial of 0.05%, 0.025%, and 0.01% Atropine Eye Drops in Myopia Control. Ophthalmol 2019;126:113-124.
- Chamberlain P, Peixoto-de-Matos SC, Logan NS et al. A 3-year Randomized Clinical Trial of MiSight Lenses for Myopia Control. Optom Vis Sci 2019;96:556-567.
- Aller TA, Liu M, Wildsoet CF. Myopia Control with Bifocal Contact Lenses: A Randomized Clinical Trial. Optom Vis Sci 2016;93:344-352.
- Lam CSY, Tang WC, Tse DY et al. Defocus Incorporated Multiple Segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomised clinical trial. Br J Ophthalmol 2020;104:363-368.
- Cheng D, Woo GC, Drobe B et al. Effect of bifocal and prismatic bifocal spectacles on myopia progression in children: three-year results of a randomized clinical trial. JAMA Ophthalmol 2014;132:258-264.













