
 This summary of an International Myopia Institute (IMI) white paper was created by and is used with permission from the Centre for Ocular Research & Education (CORE) at the University of Waterloo’s School of Optometry & Vision Science in Waterloo, Ontario, Canada. It originally appeared in the April 2019 issue of Contact Lens Update, CORE’s free access online resource.
This summary of an International Myopia Institute (IMI) white paper was created by and is used with permission from the Centre for Ocular Research & Education (CORE) at the University of Waterloo’s School of Optometry & Vision Science in Waterloo, Ontario, Canada. It originally appeared in the April 2019 issue of Contact Lens Update, CORE’s free access online resource.

The narrative surrounding genetics and refractive error has grown steadily over time, particularly with technological advances in analytical techniques and new approaches of looking at large datasets with international collaborations, including data from direct-to-consumer genetic testing company, 23andMe. Almost 200 genes have been identified in common refractive error and syndromic myopia (i.e. myopia associated with at least one other medical condition – e.g. Stickler’s syndrome), with approximately 10% of genes overlapping across these classifications (Figure 1). Sites of gene expression include all retinal cell types, RPE, vascular bed and connective tissue. Common myopia is likely to be caused by multiple genes, with each gene contributing a small effect to the overall risk of myopia. Taking all genetic risk variants to generate a polygenic risk score, a person with a high genetic load is >40 times more likely to become myopic.
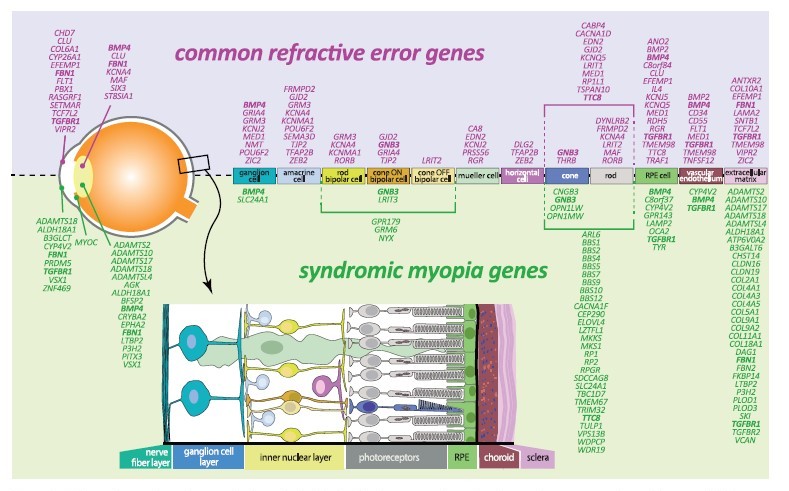 Figure 1: Schematic overview of expression in retinal cells of refractive error and syndromic myopia genes according to literature. Bold: genes identified for both common refractive error and in syndromic myopia.
Figure 1: Schematic overview of expression in retinal cells of refractive error and syndromic myopia genes according to literature. Bold: genes identified for both common refractive error and in syndromic myopia.
Diagram from sourced from Tedja MS, et al. IMI – Myopia Genetics Report.1
Studying the genetics of myopia has allowed us to gain a better understanding of the molecules and pathways involved in the disease process and eye growth. Through genome-wide pathway analyses (GWAS), several genes involved in neurotransmission, ion transport, retinoic acid metabolism, extracellular matrix modulation and eye development have been identified. In addition, gene sets involved with the detection of light stimuli and regulation of cell-cycles and growth pathways have also been identified. The Committee summarize that these findings indicate a light-induced retina-to-sclera signaling pathway in the development of myopia but the exact mechanisms by which this occurs is unknown. Genetic expression can be modified without changes to DNA sequence. The study of these changes is known as epigenetics. While epigenetics has been used extensively to study other physiological and disease processes such as cancer and cognitive dysfunction, studying epigenetic changes in ocular structures remain a challenge. Epigenetic changes are tissue- and time-specific, so it is necessary to examine the correct tissue at the correct developmental stage. Further advances in sequencing technologies and epigenetic profiling platforms are also required. In the meantime, studies have turned to examine non-coding RNAs, microRNAs (miRNA), responsible for posttranscriptional processes and epigenetic regulation. Case-control studies have identified several miRNA which mean these could become potential targets in the prevention or treatment of myopia, however not all of these findings have been supported by functional studies. The Committee emphasize that the myopia epidemic is unlikely to be caused by genetic factors, however the degree of myopia may still be under genetic control. Of particular interest are genome-environment-wide interaction studies which found subjects with a high genetic load (for myopia) and high education levels had a significantly greater risk of myopia than subjects with only one of these factors. Despite increased capabilities to examine different genetic aspects in myopia development many unknowns remain. For example, more than 150 common variants for refractive error have been identified, but this only explains approximately 8% of phenotypic variance in refractive error. Larger sample sizes are required, with special considerations for examining subgroups with extreme phenotypes and high familial occurrence. Large-scale genetic studies need to be expanded to further identify the molecular pathways involved in gene-environment effects. Improved understanding of these mechanisms and pathways in addition to the ability to identify at-risk individuals may lead to the development of targeted therapeutic treatments.
REFERENCES
- Tedja MS, Haarman AEG, Meester-Smoor MA, et al. IMI – Myopia Genetics Report. Invest Ophthalmol Vis Sci. 2019;60:M89–M105.
Other CORE summaries of IMI White Papers
All the International Myopia Institute White Papers
CORE’s Contact Lens Update, a free online resource











