

This summary of an International Myopia Institute (IMI) white paper was created by and is used with permission from the Centre for Ocular Research & Education (CORE) at the University of Waterloo’s School of Optometry & Vision Science in Waterloo, Ontario, Canada. It originally appeared in the April 2019 issue of Contact Lens Update, CORE’s free access online resource.
 The purpose of this review was to undertake a critical analysis of published papers and guidance documents, with a view to carefully considering the ethical standards associated with the investigation, development, registration, marketing, prescription and use of myopia control (MC) treatments. The review describes in detail the roles and responsibilities of a wide variety of stakeholders, including regulatory bodies, manufacturers, academics, eye care practitioners (ECPs) and patients. Particular attention is given to the ethical considerations for deciding whether to implement a myopia control strategy and how to implement this within a clinical trial or practice setting. Finally, the responsibilities in marketing, support and education required to transfer required knowledge and skills to eye care practitioners and academics are discussed.
The purpose of this review was to undertake a critical analysis of published papers and guidance documents, with a view to carefully considering the ethical standards associated with the investigation, development, registration, marketing, prescription and use of myopia control (MC) treatments. The review describes in detail the roles and responsibilities of a wide variety of stakeholders, including regulatory bodies, manufacturers, academics, eye care practitioners (ECPs) and patients. Particular attention is given to the ethical considerations for deciding whether to implement a myopia control strategy and how to implement this within a clinical trial or practice setting. Finally, the responsibilities in marketing, support and education required to transfer required knowledge and skills to eye care practitioners and academics are discussed.
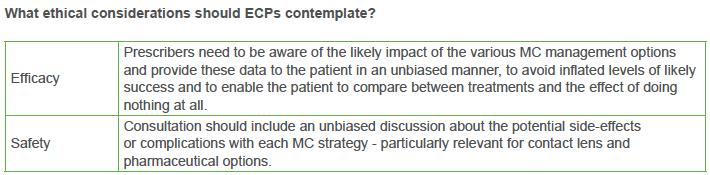
There are a variety of important ethical considerations to reflect upon when undertaking MC treatments on vulnerable populations. Some of these considerations for ECPs, industry and researchers and educators are summarised in the tables below.
For ECPs, it is essential to provide appropriate information to patients who are at risk of developing myopia or for whom myopia-related pathology could occur due to rapidly progressing myopia. An ability to convey these potential risks, in an unbiased and unemotional way, is the first step in ensuring that children and parents understand why consideration of undertaking MC management is of importance. These considerations place a burden of responsibility on the practitioner to be fully cognizant of the risks for the patient of developing different levels of myopia, the implications that progression to higher levels of myopia may have, the likely benefits of treatment, the side-effects of treatment, and other associated factors, so as to provide appropriate advice and care.

In conclusion, undertaking myopia control treatment in minors creates an ethical challenge for a wide variety of stakeholders. Regulatory bodies, manufacturers, academics and clinicians all share an ethical responsibility to ensure that the products used for myopia control are safe and efficacious and that patients understand the benefits and potential risks of such products. This IMI report highlights these ethical challenges and provides stakeholders with issues to consider in the development, financial support, prescribing and advertising of such treatments.
Other CORE summaries of IMI white papers
CORE’s Contact Lens Update, a free online resource











